Abstract
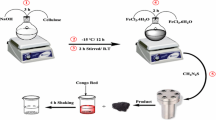
In this study, poly (ε-caprolactone)/Fe3O4 magnetic nanocomposite (PCL/Fe3O4 MNC) was synthesized by ring-opening polymerization. Then, PCL/Fe3O4 MNC was used as a novel adsorbent to remove remazol brilliant violet 5R (RBV 5R) from the aqueous media. The effects of PCL/Fe3O4 MNC dosage, process time, and initial RBV 5R concentration on the removal of RBV 5R were studied using the central composite design (CCD) method. The adsorption process was optimized using the response surface methodology, and the optimum conditions were defined. Optimum RBV 5R removal of 95.40% was obtained at a contact time of 167.98 min, PCL/Fe3O4 MNC dosage of 0.40 g, and RBV 5R concentration of 7.18 mg L−1. The process was also designed using the adsorption kinetic models and isotherm. The experimental data of RBV 5R adsorption on PCL/Fe3O4 MNC followed the Langmuir isotherm and pseudo-second-order kinetic models than the other models. Morphological and chemical properties of the adsorbent-adsorbate system indicated the high adsorption yield of RBV 5R on PCL/Fe3O4 MNC was achieved. The structural characteristics of the PCL/Fe3O4 MNC were analyzed by Fourier-transform infrared, attenuated total-reflectance (ATR), and X-ray diffraction. ATR tests showed that no significant change in chemical bond is found in PCL/Fe3O4 MNC before and after the adsorption process. The morphology of composites was investigated by scanning electron microscopy. A vibrating sample magnetometer (VSM) was used for the measurement of the magnetic property. Thermal analysis of MNC was studied using thermogravimetric analysis and differential scanning calorimetry instruments.









Similar content being viewed by others
Data Availability
All relevant data are within the manuscript.
References
Tiefenauer L, Tschirky A, Kühne G, Andres R (1996) In vivo evaluation of magnetite nanoparticles for use as a tumor contrast agent in MRI. Magn Reson Imaging 14(4):391–402
Zhang Y, Kohler N, Zhang M (2002) Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 23(7):1553–1561
Zborowski M, Sun L, Moore LR, Williams PS, Chalmers JJ (1999) Continuous cell separation using novel magnetic quadrupole flow sorter. J Magn Magn Mater 194(1–3):224–230
Lu AH, EeL S, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46(8):1222–1244
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18):3995–4021
Pekdemir ME, Ertürkan D, Külah H, Boyacı İH, Özgen C, Tamer U (2012) Ultrasensitive and selective homogeneous sandwich immunoassay detection by surface enhanced raman scattering (SERS). Analyst 137(20):4834–4840
Griffith L (2000) Polymeric biomaterials. Acta Mater 48(1):263–277
Arrieta M, Peponi L (2017) Polyurethane based on PLA and PCL incorporated with catechin: structural, thermal and mechanical characterization. Eur Polym J 89:174–184
Zhang Y, Zhuo R-x (2005) Synthesis and in vitro drug release behavior of amphiphilic triblock copolymer nanoparticles based on poly (ethylene glycol) and polycaprolactone. Biomaterials 26(33):6736–6742
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci 35(10):1217–1256
Su T-T, Jiang H, Gong H (2008) Thermal stabilities and the thermal degradation kinetics of poly (ε-caprolactone). Polym-Plast Technol Eng 47(4):398–403
Hedayatnasab Z, Dabbagh A, Abnisa F, Daud WMAW (2020) Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur Polym J 133:109789
Shen J (2006) Application of nanoparticles in polymeric foams. The Ohio State University
Wang G, Yang S, Wei Z, Dong X, Wang H, Qi M (2013) Facile preparation of poly (ε-caprolactone)/Fe 3 O 4@ graphene oxide superparamagnetic nanocomposites. Polym Bull 70(8):2359–2371
Li S, Qin J, Fornara A, Toprak M, Muhammed M, Kim DK (2009) Synthesis and magnetic properties of bulk transparent PMMA/Fe-oxide nanocomposites. Nanotechnology 20(18):185607
Wang H, Wang R, Wang L, Tian X (2011) Preparation of multi-core/single-shell OA-Fe3O4/PANI bifunctional nanoparticles via miniemulsion polymerization. Colloids Surf A 384(1–3):624–629
Zhong W, Liu P, Shi H, Xue D (2010) Ferroferric oxide/polystyrene (Fe3O4/PS) superparamagnetic nanocomposite via facile in situ bulk radical polymerization. Express Polym Lett 4(3):183–187
Barakat M (2011) Adsorption and photodegradation of procion yellow H-EXL dye in textile wastewater over TiO2 suspension. J Hydro-environ Res 5(2):137–142
Blanco SPDM, Scheufele FB, Módenes AN, Espinoza-Quiñones FR, Marin P, Kroumov AD, Borba CE (2017) Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem Eng J 307:466–475
Aguayo-Villarreal IA, Ramírez-Montoya LA, Hernández-Montoya V, Bonilla-Petriciolet A, Montes-Morán MA, Ramírez-López E (2013) Sorption mechanism of anionic dyes on pecan nut shells (Carya illinoinensis) using batch and continuous systems. Ind Crops Prod 48:89–97
Zahrim AY, Tizaoui C, Hilal N (2011) Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: a review. Desalination 266(1–3):1–16
Pajootan E, Arami M, Mahmoodi NM (2012) Binary system dye removal by electrocoagulation from synthetic and real colored wastewaters. J Taiwan Inst Chem Eng 43(2):282–290
Gök Ö, Özcan AS, Özcan A (2010) Adsorption behavior of a textile dye of reactive blue 19 from aqueous solutions onto modified bentonite. Appl Surf Sci 256(17):5439–5443
Box G (1981) Citation classic-on the experimental attainment of optimum conditions. Curr Contents/Eng Technol Appl Sci 43:20–20
Tanyildizi MŞ (2011) Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem Eng J 168(3):1234–1240
Ghosh A, Das P, Sinha K (2015) Modeling of biosorption of Cu (II) by alkali-modified spent tea leaves using response surface methodology (RSM) and artificial neural network (ANN). Appl Water Sci 5(2):191–199
Igwegbe CA, Mohmmadi L, Ahmadi S, Rahdar A, Khadkhodaiy D, Dehghani R, Rahdar S (2019) Modeling of adsorption of methylene blue dye on Ho-CaWO4 nanoparticles using response surface methodology (RSM) and artificial neural network (ANN) techniques. MethodsX 6:1779–1797
Onu CE, Nwabanne JT, Ohale PE, Asadu CO (2021) Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. S Afr J Chem Eng 36:24–42
Mansour Ghaffari M, Mostafa K (2011) Comparison of response surface methodology and artificial neural network in predicting the microwave-assisted extraction procedure to determine zinc in fish muscles. Food Nutr Sci 2011:1
Taheri M, Moghaddam MRA, Arami M (2012) Optimization of acid black 172 decolorization by electrocoagulation using response surface methodology. Iran J Environ Health Sci Eng 9(1):1–8
Mohammad Y, Shaibu-Imodagbe E, Igboro S, Giwa A, Okuofu C (2014) Modeling and optimization for production of rice husk activated carbon and adsorption of phenol. J Eng 2014:1
Cheraghipour E, Pakshir M (2021) Environmentally friendly magnetic chitosan nano-biocomposite for Cu (II) ions adsorption and magnetic nano-fluid hyperthermia: CCD-RSM design. J Environ Chem Eng 9(2):104883
Cheraghipour E, Pakshir M (2020) Process optimization and modeling of Pb (II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 260:127560
Yılmaz Ş, Zengin A, Akbulut Y, Şahan T (2019) Magnetic nanoparticles coated with aminated polymer brush as a novel material for effective removal of Pb (II) ions from aqueous environments. Environ Sci Pollut Res 26(20):20454–20468
Pekdemir ME, Coşkun M (2020) Chemical bonding of Fe 3 O 4 nanoparticles on the surface of poly (acryloyl chloride) functionalized multiwalled carbon nanotubes. Iran J Sci Technol Trans A 44(4):1001–1010
Tamer U, Gündoğdu Y, Boyacı İH, Pekmez K (2010) Synthesis of magnetic core–shell Fe 3 O 4–Au nanoparticle for biomolecule immobilization and detection. J Nanopart Res 12(4):1187–1196
Azargohar R, Dalai A (2005) Production of activated carbon from Luscar char: experimental and modeling studies. Microporous Mesoporous Mater 85(3):219–225
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977
Cui L, Xiong Z, Guo Y, Liu Y, Zhao J, Zhang C, Zhu P (2015) Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohyd Polym 132:330–337
Zhao Y, Chen Y, Zhao J, Tong Z, Jin S (2017) Preparation of SA-g-(PAA-co-PDMC) polyampholytic superabsorbent polymer and its application to the anionic dye adsorption removal from effluents. Sep Purif Technol 188:329–340
Pekdemir ME (2021) Thermal properties and shape memory behavior of titanium carbide reinforced poly (vinyl chloride)/poly (ε-caprolactone) blend nanocomposites. Polym Plast Technol Mater 61:1–9
Pekdemir ME (2021) Synthesis, characterization and thermal behavior of carbon fiber reinforced poly (vinyl chloride) and poly (ε-Caprolactone). Fibers Polym 10:1–8
İlboğa S, Pekdemir E, Coşkun M (2019) Cloud point temperature, thermal and dielectrical behaviors of thermosensitive block copolymers based N-isopropylacrylamide. Polym Sci Ser B 61(1):32–41
Pekdemir ME, Pekdemir S, İnci Ş, Kırbağ S, Çiftci M (2021) Thermal, magnetic properties and antimicrobial effects of magnetic iron oxide nanoparticles treated with Polygonum cognatum. Iran J Sci Technol Trans A 45:1–8
Pekdemir ME, Tukur A, Coskun M (2021) Thermal and dielectric investigation of magnetic nanoparticles functionalized with PVC via click chemistry. Polym Bull 78(10):6047–6057
Torğut G, Tanyol M, Meşe Z (2020) Modeling and optimization of indigo carmine adsorption from aqueous solutions using a novel polymer adsorbent: RSM-CCD. Chem Eng Commun 207(8):1157–1170
Tanyol M, Kavak N, Torğut G (2019) Synthesis of poly (AN-co-VP)/zeolite composite and its application for the removal of brilliant green by adsorption process: kinetics, isotherms, and experimental design. Adv Polym Technol 2019:1
Pekdemir ME, Pekdemir S, İnci Ş, Kırbağ S, Çiftci M (2021) Thermal, magnetic properties and antimicrobial effects of magnetic iron oxide nanoparticles treated with Polygonum cognatum. Iran J Sci Technol Trans A 45(5):1579–1586
Woo K, Hong J, Choi S, Lee H-W, Ahn J-P, Kim CS, Lee SW (2004) Easy synthesis and magnetic properties of iron oxide nanoparticles. Chem Mater 16(14):2814–2818
Pekdemir ME (2020) Poli (Vinil klorür)/Fe3o4 manyetik nanopartikül kompozitlerinin sentezi, termal ve elektriksel özelliklerinin incelenmesi. Afyon Kocatepe Üniversitesi Fen Ve Mühendislik Bilimleri Dergisi 20(5):802–809
Pekdemir ME, Qader IN, Öner E, Aydoğmuş E, Kök M, Dağdelen F (2021) Investigation of structure, mechanical, and shape memory behavior of thermally activated poly (ε-caprolactone): azide-functionalized poly (vinyl chloride) binary polymer blend films. Eur Phys J Plus 136(8):1–14
Pekdemir ME, Öner E, Kök M, Qader IN (2021) Thermal behavior and shape memory properties of PCL blends film with PVC and PMMA polymers. Iran Polym J 30(6):633–641
Mohammadi R, Mohammadifar MA, Mortazavian AM, Rouhi M, Ghasemi JB, Delshadian Z (2016) Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem 190:186–193
Venkatesa P, Girma G, Gizachew A, Surafel B, Ramesh G (2019) Biosolubilization of Cr (VI) from tannery sludge: process modeling, optimization, rate kinetics and thermodynamics aspects. Int J Recent Technol Eng 8(4):4808–4816
Yang ZK, Teng Y, Xia J, Du P (2013) Nickel oxide nanoflowers: formation, structure, magnetic property and adsorptive performance towards organic dyes and heavy metal ions. J Mater Chem A 1(31):8731–8736
Şahan T, Öztürk D (2014) Investigation of Pb (II) adsorption onto pumice samples: application of optimization method based on fractional factorial design and response surface methodology. Clean Technol Environ Policy 16(5):819–831
Bagheri R, Ghaedi M, Asfaram A, Dil EA, Javadian H (2019) RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: kinetic and isotherm studies. Polyhedron 171:464–472
Malik PK (2003) Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of Acid Yellow 36. Dyes Pigment 56(3):239–249
Draoua Z, Harrane A, Adjdir M (2021) Preparation, characterization and application of the nanocomposite PCL-PEG-PCL/Bentonite for the removal of methylene blue (MB) dye. Res Chem Intermed 47:1–21
Toprak A, Hazer B (2020) Novel porous carbon microtubes and microspheres produced from poly (CL-b-VbC) triarm block copolymer as high performance adsorbent for dye adsorption and separation. J Mol Liq 314:113565
Tanyol M, Torğut G (2021) Chitosan-graft-poly (N-Tert-Butylacrylamide) copolymer: synthesis, characterization and optimization of tetracycline removal using RSM. J Polym Environ 30:752
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Allen S, Mckay G, Porter JF (2004) Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J Colloid Interface Sci 280(2):322–333
Moon H, Lee WK (1983) Intraparticle diffusion in liquid-phase adsorption of phenols with activated carbon in finite batch adsorber. J Colloid Interface Sci 96(1):162–171
Rápó E, Aradi LE, Szabó Á, Posta K, Szép R, Tonk S (2020) Adsorption of remazol brilliant violet-5R textile dye from aqueous solutions by using eggshell waste biosorbent. Sci Rep 10(1):1–12
Bello OS, Siang TT, Ahmad MA (2012) Adsorption of remazol brilliant violet-5R reactive dye from aqueous solution by cocoa pod husk-based activated carbon: kinetic, equilibrium and thermodynamic studies. Asia-Pac J Chem Eng 7(3):378–388
Lai HJ (2021) Adsorption of remazol brilliant violet 5R (RBV-5R) and remazol brilliant blue R (RBBR) from aqueous solution by using agriculture waste. Trop Aquat Soil Pollut 1(1):11–23
Chang TW, Hadibarata T, Syafrudin M (2020) Functionalized stink bean pod (Parkia speciosa) powder for adsorption of reactive dye. Biointerface Res Appl Chem 11:11616
Rahmaniah G, Mahdi C, Safitri A (2019) Biosorption of synthetic dye from batik wastewater using Trichoderma viride immobilized on Ca-alginate. J Phys 1374:012007
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MEP synthesized, characterized and studied the magnetic properties of the polymer nanocomposite and drew all relevant graphs. MT designed all the adsorption experiments. GT and MT performed the adsorption experiments, applied the RSM technique and drew all the related graphs and tables. All authors contributed to the writing of the article, read, and reviewed the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical Approval
Not applicable.
Consent to Participate
All authors participated in the work.
Consent to Publish
All authors agree to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pekdemir, M.E., Tanyol, M. & Torğut, G. Preparation of ε-Caprolactone/Fe3O4 Magnetic Nanocomposite and Its Application to the Remazol Brilliant Violet 5R Dye Adsorption from Wastewaters by Using RSM. J Polym Environ 30, 4225–4237 (2022). https://doi.org/10.1007/s10924-022-02500-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02500-7