Abstract
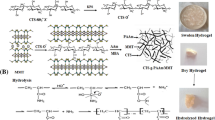
The removal of dye pollutants from dyeing and printing industrial wastewater is a very important issue, and different methods have developed for this purpose. Here, a hydrogel adsorbent gelatin/chitosan/β-cyclodextrin/sodium humate (GEL/CS/β-CD/SH) was developed by a simple method for removal of the reactive dyes in aqueous solutions. The hydrogel used as an adsorbent had a good adsorption to different kinds of organic dyes. In this work, we focused on the adsorption process of GEL/CS/β-CD/SH hydrogels to the cationic dyes methylene blue (MB) and anionic dyes acid fuchsin (AF), which are commonly used in the printing and dyeing industry. The effects of dye solution pH, ionic strength, temperature, and contact time were investigated in a batch system. The adsorption thermodynamic and dynamic behaviors towards AF and MB were investigated in detail. The adsorption process could occur spontaneously at environmental temperature. The results indicated that the maximum adsorption capacity of AF and MB reached 1666.7 mg/g and 714.3 mg/g, respectively. Moreover, the GEL/CS/β-CD/SH hydrogel could be degraded completely by microorganisms in the soil. Therefore, GEL/CS/β-CD/SH hydrogel has a potential application as an effective adsorbent for the treatment of dyeing wastewater.









Similar content being viewed by others
References
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: A review. Bioresour Technol 97:1061–1085
Pan B, Pan B, Zhang W, Zhang Q, Zhang Q, Zheng S (2008) Adsorptive removal of phenol from aqueous phase by using a porous acrylic ester polymer. J Hazard Mater 157(2–3):293–299
Umbuzeiro GA, Freeman HS, Warren SH, Oliveira DP, Terao Y, Watan-abe T, Claxton LD (2005) The contribution of azo dyes to the mutagenic activity of the Cristais River. Chemosphere 60(1):55–64
Wang Y, Chen D, Wang Y, Huang F, Hu Q, Lin Z (2012) Tunable surface charge of ZnS: Cu nano-adsorbent induced the selective preconcentration of cationic dyes from wastewater. Nanoscale 4(12):3665–3668
Thakur VK, Voicu SI (2016) Recent advances in cellulose and chitosan based membranes for water purification: a concise review. Carbohydr Polym 146:148–165
van Voorthuizen E, Zwijnenburg A, van der Meer W, Temmink H (2008) Biological black water treatment combined with membrane separation. Water Res 42(16):4334–4340
Mishra DD, Tan G (2018) Visible photocatalytic degradation of methylene blue on magnetic SrFe12O19. J Phys Chem Solids 123:157–161
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1(6):1992–2001
Sanchez AG, Ayuso EA (2002) Sorption of Zn, Cd and Cr on calcite: Application to purification of industrial wastewater. Min Eng J 15(7):539–547
Sharma G, Pathania D, Naushad M (2015) Preparation, characterization, and ion exchange behavior of nanocomposite polyaniline zirconium(IV) selenotungstophosphate for the separation of toxic metal ions. Ionics 21(4):1045–1055
Sharma G, Kumar A, Naushad M, Thakur B, Stadler FJ (2021) Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J Hazard Mater 416:125714
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemiclectrochemical and biological methods for aqueous Cr(VI) reduction.J. Hazard. Mater. 223–224, 1–12
Xu J, Chen L, Qu H, Jiao Y, Xie J, Xing G (2014) Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H3PO4. Appl Surf Sci 320:674–680
Ding J, Li B, Liu Y, Yan X, Zeng S, Zhang X, Hou L, Cai Q (2015) Zhang. Fabrication of Fe3O4 @reduced graphene oxide composite via novel colloid electrostatic selfassembly process for removal of contaminants from water. J Mater Chem A 3(2):832–839
Chaari I, Fakhfakh E, Medhioub M, Jamoussi F (2019) Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J Mol Struct 1179:672–677
Araki S, Li T, Li K, Yamamoto H (2019) Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water. Sep Purif Technol 221:393–398
Fan JC, Shi ZX, Lian M, Li H, Yin J (2013) Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J Mater Chem A 1:7433–7443
Wang Y, Yu L, Wang R, Wang Y, Zhang X (2020) A novel cellulose hydrogel coating with nanoscale Fe0 for Cr (VI) adsorption and reduction. Sci Total Environ 726:1386255
Sharma R, Kaith BS, Kalia S, Pathania D, Kumar A, Sharma N, Street RM, Schauer C (2015) Biodegradable and conducting hydrogels based on Guar gum polysaccharide for antibacterial and dye removal applications. J Environ Manag 162:37–45
Tu H, Yu Y, Chen J, Shi XW, Zhou JL, Deng HB, Du YM (2017) Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: chitosan and cellulose. Polym Chem 8(19):2913–2921
Njuguna DG, Schönherr H (2021) Xanthan Gum hydrogels as high-capacity adsorbents for dye removal. ACS Appl Polym Mater 3(6):3142–3152
Zhao X, Wang X, Lou T (2021) Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J Hazard Mater 403:124054
Hong GB, Yu TJ, Lee HC, Ma CM (2021) Using rice bran hydrogel beads to remove dye from aqueous solutions. Sustainability 13(10):1–13
Pérez-Calderón J, Santos MV, Zaritzky N (2018) Reactive red 195 dye removal using chitosan coacervated particles as bio-sorbent: Analysis of kinetics, equilibrium and adsorption mechanisms. J Environ Chem Eng 6(5):6749–6760
Liu S, Li L (2018) Unique gelation of chitosan in an alkali/urea aqueous solution. Polymers 141(8):124–131
Sujin Lim D, Jeong M-R, Ki SP, Pack YS, Choi Tyrosinase-mediated rapid and permanent chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogel.Korean J. Chem. Eng. 38(1):98–103
Ishak WHW, Ahmad I, Ramli S, Amin MCIM (2018) Gamma irradiation-assisted synthesis of cellulose nanocrystal-reinforced gelatin hydrogels. Nanomaterials 8(10):749. DOI: https://doi.org/10.3390/nano8100749
Zhang J, Xue Y, Gao F, Huang S, Zhuo R (2008) Preparation of temperature-sensitive poly(N-isopropylacrylamide)/β-cyclodextrin-grafted polyethylenimine hydrogels for drug delivery. J Appl Polym Sci 108:3031–3037
Morin-Crini N, Crini G (2013) Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog Polym Sci 38(2):344–368
Kyzas GZ, Bikiaris DN, Lambropoulou DA (2017) Effect of humic acid on pharmaceuticals adsorption using sulfonic acid grafted chitosan. J Mol Liq 230:1–5
Wang F, Sun H, Ren X, Liu Y, Zhu H, Zhang P, Ren C (2017) Effects of humic acid and heavy metals on the sorption of polar and apolar organic pollutants onto biochars. Environ Pollut 231:229–236
Nascimento FH, Masini JS (2014) Influence of humic acid on adsorption of Hg(II) by vermiculite. J Environ Manage 143:1–7
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44(16):6189–6195
Yuan Z, Liu H, Wu H, Wang Y, Wang J, Hydrogels C (2020) Rapid Removal of Aromatic Micropollutants and Adsorption Mechanisms. J Chem Eng Data 65(2):678–689
Ren J, Wang XM, Zhao LL, Li M, Yang W (2021) Double Network Gelatin/Chitosan Hydrogel Effective Removal of Dyes from Aqueous Solutions. J Polym Environ 29. DOI: https://doi.org/10.1007/s10924-021-02327-8
Nickerson MT, Patel J, Heyd DV, Rousseau D, Paulson AT (2006) Kinetic and mechanistic considerations in the gelation of genipin-crosslinked gelatin. Int J Bio Macromol 39(4–5):298–302
Santoso UT, Santosa SJ, Siswanta D, Rusdiarso B, Shimazu S (2010) Characterization of sorbent produced through immobilization of humic acid on chitosan using glutaraldehyde as cross-linking agent and Pb(II) ion as active site protector. Indo J Chem 10:301–309
Asing J, Wong NC, Lau S (2009) Optimization of extraction method and characterization of humic acid derived from coals and composts. J Trop Agric & Fd Sc 37:211–223
Santos AMP, Bertoli AC, Borges ACCP, Gomes RAB, Garcia JS, Trevisan MG (2018) New organomineral complex from humic substances extracted from poultry wastes: Synthesis, characterization and controlled release study. J Braz Chem Soc 29:140–150
Doshi B, Ayati A, Tanhaei B, Repo E, Sillanpaa M (2018) Partially carboxymethylated and partially cross-linked surface of chitosan versus the adsorptive removal of dyes and divalent metal ions. Carbohydr Polym 197:586–597
Li KR, Yan JH, Zhou Y, Li BD, Li X (2021) β-cyclodextrin and magnetic graphene oxide modified porous composite hydrogel as a superabsorbent for adsorption cationic dyes: Adsorption performance, adsorption mechanism and hydrogel column process investigates. J Mol Liq 335:116291
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529:190–194
Bhatnagar A, Sillanpää M (2009) Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater–A short review. Adv Colloid & Interface Sci 152(1–2):26–38
Deng W, Tang S, Zhou X, Liu Y, Liu S, Luo J (2020) Honeycomb-like structure-tunable chitosan-based porous carbon microspheres for methylene blue efficient removal. Carbohydr Polym 247:116736
Zheng X, Ruan Q, Jiang Q, Wang K, Wang Q, Tang Y, Huang H, Zhong C (2018) Integrated adsorption and catalytic degradation of safranine T by a porous covalent triazine-based framework. J Colloid Interface Sci 532:1–11
Hu XS, Liang R, Sun G (2018) Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J Mater Chem A 6:17612–17624
Sharma K, Kaith BS, Kumar V, Kalia S, Kumar V, Swart HC (2014) Water retention and dye adsorption behavior of Gg-cl-poly(acrylic acid-aniline) based conductive hydrogels. Geoderma 232:45–55
Joseph L, Zaib QA, Khan I, Berge ND, Park Y, Saleh NB, Yoon Y (2011) Removal of bisphenol A and 17a-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res 45:4056–4068
Shan M, Liu C, Shi L, Zhang L, Lin Y, Zhang S, Zhu Z, Wang X, Zhuang X (2019) In situ synthesis of au nanoparticles on viscose cellulose sponges for antibacterial activities,Polymers, 11(8)
Anbinder PS, Macchi C, Amalvy J, Somoza A (2019) A study of the structural changes in a chitosan matrix produced by the adsorption of copper and chromium ions,Carbohydr. Polym.,222
Dada AO, Olalekan AP, OLatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin – Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+. Unto Phosphoric Acid. Modif. Rice Husk. J Appl Chem 3:38–45
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Xu X, Yu J, Liu C, Yang G, Shi L, Zhuang X (2021) Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React & Funct Polym 160:104840
Wang N, Xu X, Li H, Zhai J, Yuan L, Zhang K, Yu H (2016) Preparation and application of a xanthate-modified Thiourea chitosan sponge for the removal of Pb (II) from aqueous solutions. Ind Eng Chem Res 55(17):4960–4968
Zhou Y, Hu X, Zhang M, Zhuo X, Niu J (2013) Preparation and characterization of modified cellulose for adsorption of Cd(II), Hg(II), and acid Fuchsin from aqueous solutions. Ind Eng Chem Res 52(2):876–884
Ho YS, Mckay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Ho YS, McKay G (1999) Pseudo-second-order model for sorption process. Process Biochem 34(5):451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Zhang R, Zhang J, Zhang X, Dou C, Han R (2014) Adsorption of Congo red from aqueous solutions using cationic surfactant modified wheat straw in batch mode: kinetic and equilibrium study. J Taiwan Inst Chem Eng 45(5):2578–2583
Zhang F, Lan J, Yang Y, Wei T, Tan R, Song W (2013) Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J Nanopart Res 15(11). https://doi.org/10.1007/s11051-013-2034-2
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Wang X, Xing Y, Zhao X, Ji Q, Xia Y, Ma X (2019) Robust, recoverable poly(N,N-dimethylacrylamide)-based hydrogels crosslinked by vinylated chitosan with recyclable adsorbability for acid red. J Appl Polym Sci 136(20):47473
Freundlich H (1932) Of the adsorption of gases. Section II. Kinetics and energetics of gas adsorption. Introductory paper to section II. Trans Faraday Soc 28:195–201
Eltaweil AS, Elgarhy GS, El-Subruiti GM, Omer AM (2020) Novel carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int J Biol Macromol 154:307–318
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133(1–3):304–308
Li DP, Zhang YR, Zhao XX, Zhao BX (2013) Magnetic nanoparticles coated by aminoguanidine for selective adsorption of acid dyes from aqueous solution. Chem Eng J 232:425–433
Zheng Y, Zhu Y, Wang A (2014) Highly efficient and selective adsorption of malachite green onto granular composite hydrogel. Chem Eng J 257:66–73
Anirudhan TS, Tharun AR (2012) Preparation and adsorption properties of a novel interpenetrating polymer network (IPN) containing carboxyl groups for basic dye from aqueous media.Chem. Eng. J.,181–182, 761–769
Bekiari V, Sotiropoulou M, Bokias G, Lianos P (2008) Use of poly(N,N-dimethylacrylamide-co-sodium acrylate) hydrogel to extract cationic dyes and metals from water. Colloids & Surf A 312(2–3):214–218
Coşkun R, Delibaş A (2012) Removal of methylene blue from aqueous solutions by poly(2-acrylamido-2-methylpropane sulfonic acid-co-itaconic acid) hydrogels. Polym Bull 68:1889–1903
Singh T, Singhal R (2012) Poly(acrylic acid/acrylamide/sodium humate) superabsorbent hydrogels for metal ion/dye adsorption: Effect of sodium humate concentration. J Appl Polym Sci 125(2):1267–1283
Yang SX, Wu YH, Wu YY, Zhu L (2015) Optimizing decolorization of Acid Fuchsin and Acid Orange II solution by MnO2 loaded MCM-41. J Taiwan Inst Chem Eng 50:205–214
Krishnan S, Chatterjee S, Solanki A, Guha N, Rai DK (2020) Aminotetrazole-functionalized SiO2 coated MgO nanoparticle composites for removal of acid fuchsin dye and detection of heavy metal ions. ACS Appl Nano Mater 3(11):11203–11216
Li Y, Sun J, Du Q, Zhang L, Yang X, Wu S, Xia Y, Wang Z, Xia L, Cao A (2014) Mechanical and dye adsorption Properties of grapheneoxide/chitosan composite fibers prepared by wet spinning. Carbohydr Polym 102:755–761
Li J, Feng J, Yan W (2013) Synthesis of polypyrrole-modified TiO2 composite adsorbent and its adsorption performance on acid Red G. J Appl Polym Sci 128(5):3231–3239
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (21665024), the Basic Project of Science and Research of Colleges and Universities of Gansu Province (5001 − 109) and the Project for Young Teacher of Northwest Normal University (NWNU-LKQN-13-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Notes
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, J., Li, R., Wang, X. et al. A superabsorbent hydrogel for removal of dyes from aqueous solution. J Polym Environ 30, 3327–3339 (2022). https://doi.org/10.1007/s10924-022-02434-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02434-0