Abstract
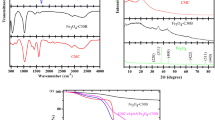
A novel biodegradable porous hydrogel (gelatin/chitosan/β-cyclodextrin, GEL/CS/β-CD) was prepared via a simple one-pot polymerization of gelatin, chitosan, and β-cyclodextrin. The chemical structure, morphology, and thermal stability were characterized by Fourier transform infrared (FT-IR), scanning electron microscopy (SEM), and thermogravimetric (TG) analysis, respectively. Due to a great number of active groups, such as amino groups, hydroxyl groups, and carboxyl groups of the hydrogel providing enough adsorption active sites, the hydrogel had excellent adsorption performances. Various organic dyes, such as malachite green (MG), crystalline violet (CV), congo red (CR), methylene blue (MB), acid fuchsin (AF), and methyl orange (MO), could be effectively removed. For 30 mg /L of MB and AF, the removal rate was between 80.0 and 93.3%. At 298 K, the adsorption capacities of AF and MB were as high as 1111 mg/g and 667 mg/g, respectively. Adsorption thermodynamics study indicated that the adsorption was a spontaneous process with a negative ΔG. The adsorption process followed the pseudo-second-order kinetic model and the Langmuir isotherm model. Moreover, the GEL/CS/β-CD hydrogel had good biodegradability and it degraded completely in soil extract solution for 30 days. Herein, the prepared GEL/CS/β-CD hydrogel has potential application as a green and environmentally friendly adsorbent for the effective removal of organic dyes in printing and dyeing wastewater.
Graphical abstract













Similar content being viewed by others
References
Li KR, Yan JH, Zhou Y et al (2021) β-cyclodextrin and magnetic graphene oxide modified porous composite hydrogel as a superabsorbent for adsorption cationic dyes: adsorption performance, adsorption mechanism, and hydrogel column process investigates. J Mol Liq 335:116291
Meng YU, Miao LIU (2019) Adsorption of dyes using multi-walled carbon nanotube hydrogel. Chem Res Chin Univ 35:311–318
Li Q, Li Y, Ma X et al (2017) Filtration and adsorption properties of porous calcium alginate membrane for methylene blue removal from water. Chem Eng J 316:623–630
Li YH, Sun JK, Du QJ et al (2014) Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning. Carbohydr Polym 102:755–761
Zahrim AY, Hilal N (2013) Treatment of highly concentrated dye solution by coagulation/flocculation-sand filtration and nanofiltration. Water Resour Ind 3:23–24
Qiu J, Feng Y, Zhang X et al (2017) Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: adsorption performance and mechanisms. J Colloid Interface Sci 499:151–158
Liu QM, Li YY, Chen HF et al (2020) Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J Hazard Mater 382:121040
Constantin M, Asmarandei I, Harabagiu V et al (2013) Removal of anionic dyes from aqueous solutions by an ion-exchanger based on pullulan microspheres. Carbohydr Polym 91:74–84
Ali I, Basheer AA, Mbianda XY, Burakov A, Galunin E, Burakova I, Mkrtchyan E, Tkachev A, Grachev V (2019) Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ Int 127:160–180
Mei SA, Gu JJ, Ma TZ et al (2019) N-doped activated carbon from used dyeing wastewater adsorbent as a metalfree catalyst for acetylene hydrochlorination. Chem Eng J 371:118–129
Sharma G, Kumar A, Naushad M et al (2018) Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohydr Polym 202:444–453
Ma SH, Yu B, Pei XW et al (2016) Structural hydrogels. Polymer 98:516–535
Thakur S, Pandey S, Arotiba OA (2016) Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr Polym 153:34–46
Mandal B, Ray SK (2016) Removal of safranine T and brilliant cresyl blue dyes from water by carboxy methyl cellulose incorporated acrylic hydrogels: isotherms, kinetics and thermodynamic study. J Taiwan Inst Chem Eng 60:313–327
Hu XS, Liang R, Sun G (2018) Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J Mater Chem A 6:17612–17624
Mohammadzadeh Pakdel P, Peighambardoust SJA (2018) review on acrylic based hydrogels and their applications in wastewater treatment. J Environ Manage 217:123–143
Hosseinzadeh H, Javadi A (2016) Fabrication and characterization of CMC-based magnetic superabsorbent hydrogel nanocomposites for crystal violet removal. Polym Adv Technol 27:1609–1616
Zhao Y, Chen Y, Zhao J et al (2017) Preparation of SA-g-(PAA-co-PDMC) polyampholytic superabsorbent polymer and its application to the anionic dye adsorption removal from effluents. Sep Purif Technol 188:329–340
Fosso-Kankeu E, Mittal H, Mishra SB et al (2015) Gum ghatti and acrylic acid based biodegradable hydrogels for the effective adsorption of cationic dyes. J Ind Eng Chem 22:171–178
Li C, Lou T, Yan X et al (2018) Fabrication of pure chitosan nanofibrous membranes as effective absorbent for dye removal. Int J Biol Macromol 106:768–774
Ren J, Wang XM, Zhao LL et al (2021) Effective removal of dyes from aqueous solutions by a gelatin hydrogel. J Polym Environ 29:3497–3508
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175–191
Ru J, Geng BY, Tong CC et al (2017) Nanocellulose-based adsorption materials Prog Chem 29:1228–1251
Zhao F, Repo E, Yin D, Meng Y et al (2015) EDTA-cross-linked beta-cyclodextrin: an environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes. Environ Sci Technol 49:10570–10580
Kono H, Onishi K, Nakamura T (2013) Characterization and bisphenol A adsorption capacity of β-cyclodextrin-carboxymethylcellulose-based hydrogels. Carbohydr Polym 98:784–792
Bae HJ, Darby DO, Kimmel RM et al (2009) Effects of transglutaminase-induced cross-linking on properties of fish gelatin–nanoclay composite film. Food Chem 114:180–189
Aranaz I, Mengibar M, Harris R et al (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
Islam S, Bhuiyan MAR, Islam MN (2017) Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ 25:854–866
Leudjo Taka A, Pillay K, Yangkou MX (2017) Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: a review. Carbohydr Polym 159:94–107
Harada A, Takashima Y, Nakahata M (2014) Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc Chem Res 47:2128–2140
Yagub MT, Sen TK, Ang HM (2012) Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut 223:5267–5282
Largitte L, Pasquier R (2016) A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des 109:495–504
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Mittal H, Al Alili A, Morajkar PP et al (2021) Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J Mol Liq 323:115034
Langmuir BI (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Hemant M, Ali AA, Saeed MA (2020) High efficiency removal of methylene blue dye using κ-carrageenan-poly(acrylamide-co-methacrylic acid)/AQSOA-Z05 zeolite hydrogel composites. Cellulose 27:8269–8285
Freundlich H (1931) Of the adsorption of gases. Section II. Kinetics and energetics of gas adsorption. Introductory paper to section II. Trans Faraday Soc 28:195–201
Hemant M, Pranay PM, Ali AA et al (2020) In-situ synthesis of ZnO nanoparticles using Gum Arabic based hydrogels as a self-template for effective malachite green dye adsorption. J Polym Environ 28:1637–1653
Melo BC, Paulinoa FAA, Cardoso VA et al (2018) Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohydr Polym 181:358-367
Teow YH, Kam LM, Mohammad AW (2018) Synthesis of cellulose hydrogel for copper(II) ions adsorption. J Environ Chem Eng 6:4588–4597
Barbetta A, Dentini M, Zannoni EM et al (2005) Tailoring the porosity and morphology of gelatin-methacrylate polyHIPE scaffolds for tissue engineering applications. Langmuir 21:12333–12341
Liu J, Liu G, Liu W (2014) Preparation of water-soluble β-cyclodextrin/poly(acrylic acid)/graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solutions. Chem Eng J 257:299–308
Doshi B, Ayati A, Tanhaei B et al (2018) Partially carboxymethylated and partially cross-linked surface of chitosan versus the adsorptive removal of dyes and divalent metal ions. Carbohydr Polym 197:586–597
Deng W, Tang S, Zhou X et al (2020) Honeycomb-like structure-tunable chitosan-based porous carbon microspheres for methylene blue efficient removal. Carbohydr Polym 247:116736
Zheng X, Ruan Q, Jiang Q et al (2018) Integrated adsorption and catalytic degradation of safranine T by a porous covalent triazine-based framework. J Colloid Interface Sci 532:1–11
Sharma K, Kaith BS, Kumar V et al (2014) Water retention and dye adsorption behavior of Gg-cl-poly(acrylic acid-aniline) based conductive hydrogels. Geoderma 232–234:45–55
Bhatnagar A, Sillanpää M (2009) Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater–a short review. Adv Colloid Interface Sci 152:26–38
Ma Y, Lv L, Guo Y et al (2017) Porous lignin based poly (acrylic acid)/organo-montmorillonite nanocomposites: swelling behaviors and rapid removal of Pb(II) ions. Polymer 128:12–23
Zheng Y, Hua S, Wang A (2010) Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 263:170–175
Mittal H, Maity A, Ray SS (2016) Gum karaya based hydrogel nanocomposites for the effective removal of cationic dyes from aqueous solutions. Appl Surf Sci 364:917–930
Mittal H, Maity A, Ray SS (2015) Synthesis of co-polymer-grafted gum karaya and silica hybrid organic-inorganic hydrogel nanocomposite for the highly effective removal of methylene blue. Chem Eng J 279:166–179
Lunkenheimer K, Geggel K, Prescher D (2017) Role of counterion in the adsorption behavior of 1:1 ionic surfactants at fluid interface-adsorption properties of alkali perfluoro-n-octanoates at the air/water interface. Langmuir 33:10216–10224
Lunkenheimer K, Prescher D, Geggel K (2022) Role of counterions in the adsorption and micellization behavior of 1:1 ionic surfactants at fluid interface-adsorption by the standard amphiphile system of alkali perfluoro-n-octanoates. Langmuir 38:891–902
Wan X, Zhan Y, Long Z et al (2017) Core@double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem Eng J 330:491–504
Wang G, Li G, Huan Y et al (2020) Acrylic acid functionalized grapheme oxide: high-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 261:127736
Manirethan V, Raval K, Rajan R et al (2018) Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. J Environ Manage 214:315–324
Huang X, Zhou D, Wang Y et al (2019) A green approach towards functional hydrogel particles from synthetic polymers via spherical capsule mini-reactors. Chem Eng J 359:1360–1371
Wu J, Li Q, Li W et al (2020) Efficient removal of acid dyes using permanent magnetic resin and its preliminary investigation for advanced treatment of dyeing effluents. J Cleaner Prod 251:119694
Zhou G, Luo J, Liu C et al (2018) Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res 131:246–254
Zhu ZB, Ouyang S, Li P et al (2020) Persistent organic pollutants removal via hierarchical flower-like layered double hydroxide: adsorption behaviors and mechanism investigation. Appl Clay Sci 188:105500
Alsbaiee A, Smith BJ, Xiao L et al (2016) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529:190–194
Zhou Y, Hu Y, Huang W et al (2018) A novel amphoteric β-cyclodextrin-based adsorbent for simultaneous removal of cationic/anionic dyes and bisphenol A. Chem Eng J 341:47–57
Jiang HL, Lin JC, Hai W et al (2019) A novel crosslinked β-cyclodextrin-based polymer for removing methylene blue from water with high efficiency. Colloids Surf A 560:59–68
Chen H, Zhou Y, Wang J et al (2020) Polydopamine modified cyclodextrin polymer as efficient adsorbent for removing cationic dyes and Cu2+. J Hazard Mater 389:121897
Huang W, Hu Y, Li Y et al (2018) Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: the roles of cavity and surface functional groups. J Taiwan Inst Chem Eng 82:189–197
Wang JW, Dai L, Liu YQ et al (2021) Adsorption properties of β-cyclodextrin modified hydrogel for methylene blue. Carbohydr Res 501:108276
Junlapong K, Maijan P, Chaibundit C et al (2020) Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int J Biol Macromol 158:258–264
Zhang C, Dai Y, Wu Y et al (2020) Facile preparation of polyacrylamide/chitosan/Fe3O4 composite hydrogels for effective removal of methylene blue from aqueous solution. Carbohydr Polym 234:115882
Huang HR, Liu ZY, Yun JH et al (2021) Preparation of Laponite hydrogel in different shapes for selective dye adsorption and filtration separation. Appl Clay Sci 201:105936
Li S, Xu J, Yao GH et al (2019) A self-adhesive, self-healable, and triple-responsive hydrogel doped with polydopamine as an adsorbent towards methylene blue. Ind Eng Chem Res 58:1–44
Singh T, Singhal R (2012) Poly(acrylic acid/acrylamide/sodium humate) superabsorbent hydrogels for metal ion/dye adsorption: effect of sodium humate concentration. J Appl Polym Sci 125:1267–1283
Paulino AT, Guilherme MR, Reis AV et al (2006) Removal of methylene blue dye from an aqueous media using superabsorbent hydrogel supported on modified polysaccharide. J Colloid Interf Sci 301:55–62
Ren J, Wang XM, Zhao LL et al (2021) Double network gelatin/chitosan hydrogel effective removal of dyes from aqueous solutions. J Polym Environ. https://doi.org/10.1007/s10924-021-02327-8
Yang SX, Wu YH, Wu YY et al (2015) Optimizing decolorization of Acid Fuchsin and Acid Orange II solution by MnO2 loaded MCM-41. J Taiwan Inst Chem Eng 50:205–214
Qin Q, Ma J, Liu K (2009) Adsorption of anionic dyes on ammonium-functionalized MCM-41. J Hazard Mater 162:133–139
Gong N, Liu YP, Huang RH (2018) Simultaneous adsorption of Cu2+ and Acid fuchsin (AF) from aqueous solutions by CMC/bentonite composite. Int J Biol Macromol 115:580–589
Li J, Feng J, Yan W (2013) Synthesis of polypyrrole-modified TiO2 composite adsorbent and its adsorption performance on acid red G. J Appl Polym Sci 128:3231–3239
Funding
This work was supported in part by National Natural Science Foundation of China (21665024), the Basic Project of Science and Research of Colleges and Universities of Gansu Province (5001–109), and the Project for Young Teacher of Northwest Normal University (NWNU-LKQN-13–6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, J., Li, M., Wang, X. et al. Adsorption behaviors of dyes on a biodegradable gelatin/chitosan/β-cyclodextrin hydrogel from an aqueous solution. Colloid Polym Sci 300, 785–800 (2022). https://doi.org/10.1007/s00396-022-04988-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-04988-w