Abstract
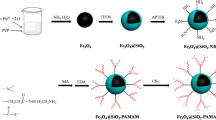
In this work, three different generations of Poly(amidoamine) dendrimers (PAMAM) decorated on magnetic Fe3O4 composites (Fe3O4@SiO2–G1, Fe3O4@SiO2–G3, Fe3O4@SiO2–G5) were fabricated and characterized by FTIR, XRD, TEM, TGA, VSM and XPS. The obtained composites were used for Cr(VI) removal. Batch adsorption studies showed that the adsorption reached equilibrium within 60 min, and the optimal pH was 3.0. The result of adsorption kinetics was simulated by the pseudo-second-order model. The adsorption equilibrium isotherm was well fitted with the Langmuir adsorption model. Furthermore, thermodynamics calculations revealed that the adsorption process was endothermic and spontaneous. Importantly, adsorption capacity of Cr(VI) obeyed the sequence of Fe3O4@SiO2–G1 < Fe3O4@SiO2–G5 < Fe3O4@SiO2–G3, 3 generation of PAMAM (G3) was the optimal for adsorption capacity of Cr(VI). The maximum theoretical Cr(VI) adsorption capacity (qm) of Fe3O4@SiO2–G3 was 334.45 mg/g, and removal ration remained above 89.5% after five cycles of adsorption–desorption. Thus, Fe3O4@SiO2–G3 is predicted to be an efficient adsorbent for the adsorption of Cr(VI) from aqueous solution, and the obtained results can help in the generation optimization during fabrication of dendrimer modified adsorbents.












Similar content being viewed by others
References
S. Chowdhury, M.A.J. Mazumder, O. Al-Attas, T. Husain, Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci. Total Environ. 569, 476–488 (2016)
M.L. Sall, A.K.D. Diaw, D. Gningue-Sall, S.E. Aaron, J.J. Aaron, Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. R 27(24), 29927–29942 (2020)
C. Donga, S.B. Mishra, A.S. Abd-El-Aziz, A.K. Mishra, Advances in graphene-based magnetic and graphene-based/TiO(2)nanoparticles in the removal of heavy metals and organic pollutants from industrial wastewater. J. Inorg. Organomet. P 31(2), 463–480 (2020)
M. Balali-Mood, K. Naseri, Z. Tahergorabi, M.R. Khazdair, M. Sadeghi, Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 643972 (2021)
S.P. Wu, X.Z. Dai, T.T. Cheng, S.J. Li, Highly sensitive and selective ion-imprinted polymers based on one-step electrodeposition of chitosan–graphene nanocomposites for the determination of Cr(VI). Carbohyd. Polym. 195, 199–206 (2018)
H. Peng, J. Guo, Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environ. Chem. Lett. 18(6), 2055–2068 (2020)
B.H. Xie, C. Shan, Z. Xu, X.C. Li, X.L. Zhang, J.J. Chen, B.C. Pan, One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 308, 791–797 (2017)
Y.F. Ren, Y.H. Han, Q.Y. Zhang, A magnetic ion exchange resin with high efficiency of removing Cr (VI). Colloid Surf. A 604, 125279 (2020)
G. Sharma, D. Pathania, M. Naushad, N.C. Kothiyal, Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: efficient removal of toxic metal ions from water. Chem. Eng. J. 251, 413–421 (2014)
W. Jin, H. Du, S.L. Zheng, Y. Zhang, Electrochemical processes for the environmental remediation of toxic Cr(VI): a review. Electrochim. Acta 191, 1044–1055 (2016)
G. Sharma, A. Kumar, M. Naushad, B. Thakur, D.V.N. Vo, B. Gao, A.A. Al-Kahtani, F.J. Stadler, Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 416, 125714 (2021)
E. Doustkhah, S. Rostamnia, H.G. Hossieni, R. Luque, Covalently bonded PIDA on SBA-15 as robust Pd support: water-tolerant designed catalysts for aqueous suzuki couplings. ChemistrySelect 2(1), 329–334 (2017)
Y.C. Zhu, C.C. Jiao, L.Q. Han, Y.H. Gao, S.J. Li, X.H. Yuan, A novel PHEMA-based bismuth oxide composite with high photocatalytic activity. J. Inorg. Organomet. P 30(11), 4739–4752 (2020)
S. Gao, Z.C. Liu, Q.S. Yan, P. Wei, Y. Li, J.Y. Ji, L. Li, Facile synthesis of polypyrrole/reduced graphene oxide composite hydrogel for Cr(VI) removal. J. Inorg. Organomet. P. 31(9), 3677–3685 (2021)
A. Mudhoo, D. Mohan, C.U. Pittman, G. Sharma, M. Sillanpaa, Adsorbents for real-scale water remediation: gaps and the road forward. J. Environ. Chem. Eng. 9(4), 105380 (2021)
D. Mohan, C.U. Pittman, Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 137(2), 762–811 (2006)
W.Z. Gu, D.C. Zheng, D.P. Li, C.C. Wei, X. Wang, Q.Z.M. Yang, C. Tian, M.Y. Cui, Integrative effect of citrate on Cr(VI) and total Cr removal using a sulfate-reducing bacteria consortium. Chemosphere 279, 130437 (2021)
Y. Song, Z.X. Li, S.J. Shao, W.Z. Jiao, Y.Z. Liu, High-gravity intensified preparation of D201 resin-hydrated iron oxide nanocomposites for Cr(VI) removal. Adv. Powder Technol. 32(5), 1584–1593 (2021)
B. Zhang, Y.H. Wu, Y. Fan, Synthesis of novel magnetic NiFe2O4 nanocomposite grafted chitosan and the adsorption mechanism of Cr(VI). J. Inorg. Organomet. P. 29(1), 290–301 (2019)
H. Alamgholiloo, S. Rostamnia, K.Q. Zhang, T.H. Lee, Y.S. Lee, R.S. Varma, H.W. Jang, M. Shokouhimehr, Boosting aerobic oxidation of alcohols via synergistic effect between TEMPO and a composite Fe3O4/Cu–BDC/GO nanocatalyst. ACS Omega 5(10), 5182–5191 (2020)
X.H. Yang, J.R. Kan, F.Y. Zhang, M.Y. Zhu, S.J. Li, Facile fabrication of Mn2+ doped magnetite microspheres as efficient electrode material for supercapacitors. J. Inorg. Organomet. P. 27(2), 542–551 (2017)
E. Doustkhah, S. Rostamnia, B. Gholipour, B. Zeynizadeh, A. Baghban, R. Luque, Design of chitosan–dithiocarbamate magnetically separable catalytic nanocomposites for greener aqueous oxidations at room temperature. Mol. Catal. 434, 7–15 (2017)
N.A. Jumat, S.H. Khor, W.J. Basirun, J.C. Juan, S.W. Phang, Highly visible light active ternary polyaniline-TiO2–Fe3O4 nanotube/nanorod for photodegradation of reactive black 5 dyes. J. Inorg. Organomet. P. 31(5), 2168–2181 (2021)
S. Rostamnia, B. Zeynizadeh, E. Doustkhah, A. Baghban, K.O. Aghbash, The use of K-carrageenan/Fe3O4 nanocomposite as a nanomagnetic catalyst for clean synthesis of rhodanines. Catal. Commun. 68, 77–83 (2015)
Q. Chen, W.J. Wei, J.J. Tang, J.K. Lin, S.J. Li, M.Y. Zhu, Dopamine-assisted preparation of Fe3O4@MnO2 yolk@shell microspheres for improved pseudocapacitive performance. Electrochim. Acta. 317, 628–637 (2019)
J.F. Liu, Z.S. Zhao, G.B. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 42(18), 6949–6954 (2008)
G. Sharma, A. Kumar, S. Sharma, M. Naushad, P. Dhiman, D.V.N. Vo, F.J. Stadler, Fe3O4/ZnO/Si3N4 nanocomposite based photocatalyst for the degradation of dyes from aqueous solution. Mater. Lett. 278, 128359 (2020)
S. Bagheri, A. Esrafili, M. Kermani, J. Mehralipour, M. Gholami, Performance evaluation of a novel rGO-Fe-0/Fe3O4-PEI nanocomposite for lead and cadmium removal from aqueous solutions. J. Mol. Liq. 320, 114422 (2020)
E. Doustkhah, M. Heidarizadeh, S. Rostamnia, A. Hassankhani, B. Kazemi, X. Liu, Copper immobilization on carboxylic acid-rich Fe3O4–Pectin: Cu2+@Fe3O4–Pectin a superparamagnetic nanobiopolymer source for click reaction. Mater. Lett. 216, 139–143 (2018)
A. Zarei, S. Saedi, F. Seidi, Synthesis and application of Fe3O4@SiO2@carboxyl-terminated PAMAM dendrimer nanocomposite for heavy metal removal. J. Inorg. Organomet. P. 28(6), 2835–2843 (2018)
M. Nourmohammadi, S. Rouhani, S. Azizi, M. Maaza, T.A.M. Msagati, S. Rostamnia, M. Hatami, S. Khaksar, E. Zarenezhad, H.W. Jang, Magnetic nanocomposite of crosslinked chitosan with 4,6-diacetylresorci-nol for gold immobilization (Fe3O4@CS/DAR–Au) as a catalyst for an efficient one-pot synthesis of propargylamine. Mater. Today Commun. 29, 102798 (2021)
F. Ke, J. Jiang, Y.Z. Li, J. Liang, X.C. Wan, S. Ko, Highly selective removal of Hg2+ and Pb2+ by thiol-functionalized Fe3O4@metal–organic framework core–shell magnetic microspheres. Appl. Surf. Sci. 413, 266–274 (2017)
N. Alhokbany, T. Ahamad, M. Naushad, S.M. Alshehri, Feasibility of toxic metal removal from aqueous medium using Schiff-base based highly porous nanocomposite: adsorption characteristics and post characterization. J. Mol. Liq. 294, 111598 (2019)
W. Fu, X.Y. Wang, Z.Q. Huang, Remarkable reusability of magnetic Fe3O4-encapsulated C3N3S3 polymer/reduced graphene oxide composite: a highly effective adsorbent for Pb and Hg ions. Sci. Total Environ. 659, 895–904 (2019)
B. Surekha, N.S. Kommana, S.K. Dubey, A.V.P. Kumar, R. Shukla, P. Kesharwani, PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloid Surf. B 204, 111837 (2021)
M. Nikzamir, Y. Hanifehpour, A. Akbarzadeh, Y. Panahi, Applications of dendrimers in nanomedicine and drug delivery: a review. J. Inorg. Organomet. P. 31(6), 2246–2261 (2021)
E.B. Bahadir, M.K. Sezginturk, Poly(amidoamine) (PAMAM): an emerging material for electrochemical bio(sensing) applications. Talanta 148, 427–438 (2016)
H. Viltres, Y.C. Lopez, C. Leyva, N.K. Gupta, A.G. Naranjo, P. Acevedo-Pena, A. Sanchez-Diaz, J. Bae, K.S. Kim, Polyamidoamine dendrimer-based materials for environmental applications: a review. J. Mol. Liq. 334, 116017 (2021)
B. Ren, K. Wang, B.S. Zhang, H.Y. Li, Y.Z. Niu, H. Chen, Z.L. Yang, X.Z. Li, H.Q. Zhang, HQ, Adsorption behavior of PAMAM dendrimers functionalized silica for Cd(II) from aqueous solution: experimental and theoretical calculation. J. Taiwan Inst. Chem. E 101, 80–91 (2019)
M. Heidarizadeh, E. Doustkhah, F. Saberi, S. Rostamnia, A. Hassankhani, P.F. Rezaei, Silica nanostructures, a heterogeneous surface for dendrimer functionalization. ChemistrySelect 3(25), 7137–7151 (2018)
Y. Mansourpanah, A. Ghanbari, H. Yazdani, A.G. Mohammadi, A. Rahimpour, Silver-polyamidoamine/graphene oxide thin film nanofiltration membrane with improved antifouling and antibacterial properties for water purification and desalination. Desalination 511, 115109 (2021)
D.K. Cheng, X.H. Dai, L. Chen, Y.H. Cui, C.W. Qiang, Q. Sun, J.D. Dai, Thiol-yne click synthesis of polyamide-amine dendritic magnetic halloysite nanotubes for the efficient removal of Pb(II). ACS Sustain. Chem. Eng. 8(2), 771–781 (2020)
L.P. Luan, B.T. Tang, Y.F. Liu, A.L. Wang, B.B. Zhang, W.L. Xu, Y.Z. Niu, Selective capture of Hg(II) and Ag(I) from water by sulfur-functionalized polyamidoamine dendrimer/magnetic Fe3O4 hybrid materials. Sep. Purif. Technol. 257, 117902 (2021)
M. Mikhaylova, D.K. Kim, C.C. Berry, A. Zagorodni, M. Toprak, A.S.G. Curtis, M. Muhammed, BSA immobilization on amine-functionalized superparamagnetic iron oxide nanoparticles. Chem. Mater. 16(12), 2344–2354 (2004)
S. Rostamnia, B. Gholipour, X. Liu, Y. Wang, H. Arandiyan, NH2-coordinately immobilized tris(8-quinolinolato)iron onto the silica coated magnetite nanoparticle: Fe3O4@SiO2–FeQ(3) as a selective Fenton-like catalyst for clean oxidation of sulfides. J. Colloid. Interf. Sci. 511, 447–455 (2018)
P. Dhiman, S. Sharma, A. Kumar, M. Shekh, G. Sharma, M. Naushad, Rapid visible and solar photocatalytic Cr(VI) reduction and electrochemical sensing of dopamine using solution combustion synthesized ZnO–Fe2O3 nano heterojunctions: mechanism elucidation. Ceram. Int. 46(8), 12255–12268 (2020)
W.M. Liu, Y.N. Xue, N. Peng, W.T. He, R.X. Zhuo, S.W. Huang, Dendrimer modified magnetic iron oxide nanoparticle/DNA/PEI ternary magnetoplexes: a novel strategy for magnetofection. J. Mater. Chem. 21(35), 13306–13315 (2011)
J. Shang, Y.N. Guo, D.L. He, W. Qu, Y.N. Tang, L. Zhou, R.L. Zhu, A novel graphene oxide-dicationic ionic liquid composite for Cr(VI) adsorption from aqueous solutions. J. Hazard. Mater. 416, 125706 (2021)
G. Sharma, B. Thakur, A. Kumar, S. Sharma, M. Naushad, F.J. Stadler, Atrazine removal using chitin-cl-poly(acrylamide-co-itaconic acid) nanohydrogel: isotherms and pH responsive nature. Carbohyd. Polym. 241, 116258 (2020)
N. Rezak, A. Bahmani, N. Bettahar, Adsorptive removal of P(V) and Cr(VI) by calcined Zn–Al–Fe ternary LDHs. Water Sci. Technol. 83(10), 2504–2517 (2021)
T.F. Guo, H.N. Gu, S.C. Ma, N. Wang, Increasing phosphate sorption on barium slag by adding phosphogypsum for non-hazardous treatment. J. Environ. Manag. 270, 110823 (2020)
J.J. Yang, Z.F. Zhang, W.T. Pang, H.J. Chen, G.Q. Yan, Polyamidoamine dendrimers functionalized magnetic carbon nanotubes as an efficient adsorbent for the separation of flavonoids from plant extraction. Sep. Purif. Technol. 227, 115710 (2019)
G. Sharma, T.S. AlGarni, P.S. Kumar, S. Bhogal, A. Kumar, S. Sharma, M. Naushad, Z.A. ALOthman, F.J. Stadler, Utilization of Ag2O–Al2O3–ZrO2 decorated onto rGO as adsorbent for the removal of Congo red from aqueous solution. Environ. Res. 197, 111179 (2021)
W.J. Shen, Y. Mu, T. Xiao, Z.H. Ai, Magnetic Fe3O4–FeB nanocomposites with promoted Cr(VI) removal performance. Chem. Eng. J. 285, 57–68 (2016)
Y. Zhang, Y.Y. Mo, T. Vincent, C. Faur, E. Guibal, Boosted Cr(VI) sorption coupled reduction from aqueous solution using quaternized algal/alginate@PEI beads. Chemosphere 281, 130844 (2021)
H.S. Ramadan, M. Mobarak, E.C. Lima, A. Bonilla-Petriciolet, Z.C. Li, M.K. Seliem, Cr(VI) adsorption onto a new composite prepared from Meidum black clay and pomegranate peel extract: experiments and physicochemical interpretations. J. Environ. Chem. Eng. 9(4), 105352 (2021)
M.B. Poudel, G.P. Awasthi, H.J. Kim, Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co–Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 417, 129312 (2021)
L.L. Du, P. Gao, Y.L. Liu, T. Minami, C.B. Yu, Removal of Cr(VI) from aqueous solution by polypyrrole/hollow mesoporous silica particles. Nanomaterials 10(4), 686 (2020)
Y. Yang, Y.H. Zhang, G.Y. Wang, Z.B. Yang, J.R. Xian, Y.X. Yang, T. Li, Y.L. Pu, Y.X. Jia, Y. Li, Z. Cheng, S.R. Zhang, X.X. Xu, Adsorption and reduction of Cr(VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J. Environ. Chem. Eng. 9, 105407 (2021)
Acknowledgements
This work is financially supported by the Science and Technology Foundation of Guizhou Province, China ([2018]1173, [2020]1Y163, [2018]1170), Key Science and Technology Support Project of Guizhou Province, China ([2021]326) and the National Natural Science Foundation of China (41803050, 52062005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cui, C., Xie, YD., Niu, JJ. et al. Poly(Amidoamine) Dendrimer Modified Superparamagnetic Nanoparticles as an Efficient Adsorbent for Cr(VI) Removal: Effect of High-Generation Dendrimer on Adsorption Performance. J Inorg Organomet Polym 32, 840–853 (2022). https://doi.org/10.1007/s10904-021-02222-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02222-8