Abstract
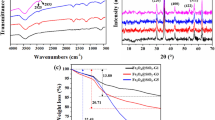
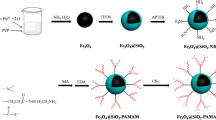
In this survey a new route has been developed the preparation of poly (amidoamine) generation 6 (PAMAM-G6) dendrimer functionalized Fe3O4/SiO2 nanoparticle and was used for arsenite (As (III)) adsorption. SiO2 was first grafted onto the surface of Fe3O4 to formation a core–shell structure. Then the introduction of epoxy rings were done by hydrolysis of methylsilane groups of 3-Glycidoxypropyltrimethoxysilane (GPTMS) on OH groups of SiO2 and afterwards, PAMAM-G6 reacted with epoxy rings of GPTMS to obtain a multiamino magnetic adsorbent. The as-prepared nanocomposite was characterized by TEM, Zeta potential, FESEM, VSM, FTIR, Raman and XPS techniques. The effects of reaction time from 5 to 50 min, initial As (III) concentration in the range of 1–10 mgL−1, initial adsorbent concentration in the range of 10–50 mgL−1 and initial pH in the range 3–8 were studied. The resulting of kinetic and isotherm models displays high adsorption affinity (233 mg/g) for As (III) and the adsorbent can reach the adsorbent can reach the adsorption equilibrium at a neutral pH (7). The As (III) loaded nanocomposite could be separated readily from aqueous solution by magnetic and regenerated simply via NaOH. The study of the adsorption procedure showed that the pseudo-second order kinetics and Langmuir isotherm well-fitted with the experimental data of As (III) adsorption onto nanocomposite.















Similar content being viewed by others
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Basel: Springer; 2012. p. 133–64.
Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat Res/Rev Mutat Res. 2006;612(3):215–46.
Tangahu BV, Abdullah S, Rozaimah S, Basri H, Idris M, Anuar N, et al. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. 2011;2011:1–31. https://doi.org/10.1155/2011/939161.
Ma M-D, Wu H, Deng Z-Y, Zhao X. Arsenic removal from water by nanometer iron oxide coated single-wall carbon nanotubes. J Mol Liq. 2018;259:369–75.
Wong W, Wong H, Badruzzaman ABM, Goh H, Zaman M. Recent advances in exploitation of nanomaterial for arsenic removal from water: a review. Nanotechnology. 2016;28(4):042001.
Cheng Y-Y, Huang N-C, Chang Y-T, Sung J-M, Shen K-H, Tsai C-C, et al. Associations between arsenic in drinking water and the progression of chronic kidney disease: a nationwide study in Taiwan. J Hazard Mater. 2017;321:432–9.
Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, et al. Synthesis and characterization of multi-amino-functionalized cellulose for arsenic adsorption. Carbohydr Polym. 2013;92(1):380–7.
Asmel NK, Yusoff ARM, Krishna LS, Majid ZA, Salmiati S. High concentration arsenic removal from aqueous solution using nano-iron ion enrich material (NIIEM) super adsorbent. Chem Eng J. 2017;317:343–55.
Ghosh A, Chakrabarti S, Ghosh UC. Fixed-bed column performance of Mn-incorporated iron (III) oxide nanoparticle agglomerates on As (III) removal from the spiked groundwater in lab bench scale. Chem Eng J. 2014;248:18–26.
Ali I. New generation adsorbents for water treatment. Chem Rev. 2012;112(10):5073–91.
AlOmar MK, Alsaadi MA, Hayyan M, Akib S, Hashim MA. Functionalization of CNTs surface with phosphonuim based deep eutectic solvents for arsenic removal from water. Appl Surf Sci. 2016;389:216–26.
Rastegar A, Alahabadi A, Esrafili A, Rezai Z, Hosseini-Bandegharaei A, Nazari S. Application of supramolecular solvent-based dispersive liquid–liquid microextraction for trace monitoring of lead in food samples. Anal Methods. 2016;8(27):5533–9.
Kavosi B, Salimi A, Hallaj R, Moradi F. Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens Bioelectron. 2015;74:915–23.
Gholami M, Nazari S, Farzadkia M, Mohseni SM, Alizadeh Matboo S, Akbari Dourbash F, et al. Nano polyamidoamine-G7 dendrimer synthesis and assessment the antibacterial effect in vitro. Tehran Univ Med J TUMS Publications. 2016;74(1):25–35.
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G. Synthesis and characterization of PAMAM/CNT nanocomposite as a super-capacity adsorbent for heavy metal (Ni2+, Zn2+, As3+, Co2+) removal from wastewater. J Mol Liq. 2016;224:1032–40.
Gholami M, Nazari S, Farzadkia M, Majidi G, Matboo SA. Assessment of nanopolyamidoamine-G7 dendrimer antibacterial effect in aqueous solution. Tehran Univ Med J. 2016;74(3):159–67.
Al-Khaldi FA, Abusharkh B, Khaled M, Atieh MA, Nasser M, Saleh TA, et al. Adsorptive removal of cadmium (II) ions from liquid phase using acid modified carbon-based adsorbents. J Mol Liq. 2015;204:255–63.
Rastegar A, Nazari S, Allahabadi A, Falanji F, Dourbash FADA, Rezai Z, et al. Antibacterial activity of amino-and amido-terminated poly (amidoamine)-G6 dendrimer on isolated bacteria from clinical specimens and standard strains. Med J Islam Repub Iran. 2017;31:64.
Valdés O, Vergara C, Nachtigall FM, Lopez-Cabaña Z, Tapia J, Santos LS. Pamam built-on-silicon wafer thin-layer extraction devices for selective metal contamination detection. Tetrahedron Lett. 2016;57(23):2468–73.
Zhao X, Shi Y, Wang T, Cai Y, Jiang G. Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J Chromatogr A. 2008;1188(2):140–7.
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D. Amino-functionalized Fe 3 O 4@ SiO 2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci. 2010;349(1):293–9.
Pasandideh EK, Kakavandi B, Nasseri S, Mahvi AH, Nabizadeh R, Esrafili A, et al. Silica-coated magnetite nanoparticles core-shell spheres (Fe 3 O 4@ SiO 2) for natural organic matter removal. J Environ Health Sci Eng. 2016;14(1):1–13.
Acres RG, Ellis AV, Alvino J, Lenahan CE, Khodakov DA, Metha GF, et al. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J Phys Chem C. 2012;116(10):6289–97.
Zhang J-M, Zhai S-R, Zhai B, An Q-D, Tian G. Crucial factors affecting the physicochemical properties of sol–gel produced Fe3O4@ SiO2–NH2 core–shell nanomaterials. J Sol-Gel Sci Technol. 2012;64(2):347–57.
Wang J, Zheng C, Ding S, Ma H, Ji Y. Behaviors and mechanisms of tannic acid adsorption on an amino-functionalized magnetic nanoadsorbent. Desalination. 2011;273(2):285–91.
Gholami M, Mohammadi R, Arzanlou M, Dourbash FA, Kouhsari E, Majidi G, et al. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect Dis. 2017;17(1):395.
Srinivasan B, Huang X. Functionalization of magnetic nanoparticles with organic molecules: loading level determination and evaluation of linker length effect on immobilization. Chirality. 2008;20(3–4):265–77.
Liu F, Niu F, Peng N, Su Y, Yang Y. Synthesis, characterization, and application of Fe 3 O 4@ SiO 2–NH 2 nanoparticles. RSC Adv. 2015;5(23):18128–36.
Feng G, Hu D, Yang L, Cui Y. Cui X-a, Li H. immobilized-metal affinity chromatography adsorbent with paramagnetism and its application in purification of histidine-tagged proteins. Sep Purif Technol. 2010;74(2):253–60.
Dourbash FA, Alizadeh P, Nazari S, Farasat A. A highly bioactive poly (amido amine)/70S30C bioactive glass hybrid with photoluminescent and antimicrobial properties for bone regeneration. Mater Sci Eng C. 2017;78:1135–46.
Nazari S, Gholami M, Farzadkia M, Dourbash FA, Arzanlou M, Kalantary RR. Synthesis and evaluation of the antibacterial effect of silica-coated modified magnetic poly-(amidoamine) G5 nanoparticles on E. coli and S. aureus. J Mol Liq. 2018;276:93–104.
Zhang Z, Li M, Chen W, Zhu S, Liu N, Zhu L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ Pollut. 2010;158(2):514–9.
Maleki A, Hayati B, Najafi F, Gharibi F, Joo SW. Heavy metal adsorption from industrial wastewater by PAMAM/TiO2 nanohybrid: preparation, characterization and adsorption studies. J Mol Liq. 2016;224:95–104.
Tan Y, Chen M, Hao Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem Eng J. 2012;191:104–11.
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J Hazard Mater. 2017;336:146–57.
Sheikhmohammadi A, Dahaghin Z, Mohseni SM, Sarkhosh M, Azarpira H, Atafar Z, et al. The synthesis and application of the SiO2@ Fe3O4@ MBT nanocomposite as a new magnetic sorbent for the adsorption of arsenate from aqueous solutions: modeling, optimization, and adsorption studies. J Mol Liq. 2018;255:313–23.
Sheikhmohammadi A, Mohseni SM, Sardar M, Abtahi M, Mahdavi S, Keramati H, et al. Application of graphene oxide modified with 8-hydroxyquinoline for the adsorption of Cr (VI) from wastewater: optimization, kinetic, thermodynamic and equilibrium studies. J Mol Liq. 2017;233:75–88.
Anirudhan TS, Senan P, Suchithra PS. Evaluation of iron (III)-coordinated amino-functionalized poly (glycidyl methacrylate)-grafted cellulose for arsenic (V) adsorption from aqueous solutions. Water Air Soil Pollut. 2011;220(1–4):101–16.
Hermanson GT. Bioconjugate techniques. Cambridge: Academic Press; 2013.
Hanim SAM, Malek NANN, Ibrahim Z. Analyses of surface area, porosity, silver release and antibacterial activity of amine-functionalized, silver-exchanged zeolite NaY. Vacuum. 2017;143:344–7.
Muraliganth T, Murugan AV, Manthiram A. Facile synthesis of carbon-decorated single-crystalline Fe3O4 nanowires and their application as high performance anode in lithium ion batteries. Chem Commun. 2009;47:7360–2.
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D. Amino-functionalized Fe3O4@ SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J Colloid Interface Sci. 2010;349(1):293–9.
Jesionowski T, Ciesielczyk F, Krysztafkiewicz A. Influence of selected alkoxysilanes on dispersive properties and surface chemistry of spherical silica precipitated in emulsion media. Mater Chem Phys. 2010;119(1):65–74.
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G. Synthesis and characterization of PAMAM/CNT nanocomposite as a super-capacity adsorbent for heavy metal (Ni 2+, Zn 2+, As 3+, Co 2+) removal from wastewater. J Mol Liq. 2016;224:1032–40.
Hayati B, Maleki A, Najafi F, Daraei H, Gharibi F, McKay G. Adsorption of Pb2+, Ni2+, Cu2+, Co2+ metal ions from aqueous solution by PPI/SiO2 as new high performance adsorbent: preparation, characterization, isotherm, kinetic, thermodynamic studies. 2017;237:428–36.
Tan P, Sun J, Hu Y, Fang Z, Bi Q, Chen Y, et al. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes. J Hazard Mater. 2015;297:251–60.
Martinson CA, Reddy K. Adsorption of arsenic (III) and arsenic (V) by cupric oxide nanoparticles. J Colloid Interf Sci. 2009;336(2):406–11.
Saleh TA, Sarı A, Tuzen M. Chitosan-modified vermiculite for As (III) adsorption from aqueous solution: equilibrium, thermodynamic and kinetic studies. J Mol Liq. 2016;219:937–45.
Niu Y, Qu R, Chen H, Mu L, Liu X, Wang T, et al. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg (II) from aqueous solution. J Hazard Mater. 2014;278:267–78.
Martinson CA, Reddy K. Adsorption of arsenic (III) and arsenic (V) by cupric oxide nanoparticles. J Colloid Interface Sci. 2009;336(2):406–11.
Saleh TA, Sarı A, Tuzen M. Chitosan-modified vermiculite for as (III) adsorption from aqueous solution: equilibrium, thermodynamic and kinetic studies. J Mol Liq. 2016;219:937–45.
Niu Y, Qu R, Chen H, Mu L, Liu X, Wang T, et al. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of hg (II) from aqueous solution. J Hazard Mater. 2014;278:267–78.
Liu H, Liu W, Zhang J, Zhang C, Ren L, Li Y. Removal of cephalexin from aqueous solutions by original and cu (II)/Fe (III) impregnated activated carbons developed from lotus stalks kinetics and equilibrium studies. J Hazard Mater. 2011;185(2–3):1528–35.
Moussavi G, Khosravi R. Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: parametric experiments, kinetics and equilibrium analysis. J Hazard Mater. 2010;183(1–3):724–30.
Acknowledgements
The authors appreciate the cooperation of students, professors, members of the expert panel, and all people who helped us throughout this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbari, H., Gholami, M., Akbari, H. et al. Poly (amidoamine) generation 6 functionalized Fe3O4@SiO2/GPTMS core–shell magnetic NPs as a new adsorbent for Arsenite adsorption: kinetic, isotherm and thermodynamic studies. J Environ Health Sci Engineer 18, 253–265 (2020). https://doi.org/10.1007/s40201-020-00461-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00461-4