Abstract
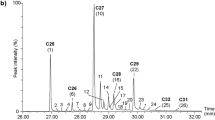
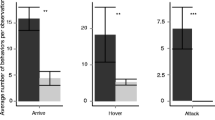
Parasitic wasps which attack insects infesting processed stored food need to locate their hosts hidden inside these products. Their host search is well-known to be guided by host kairomones, perceived via olfaction or contact. Among contact kairomones, host cuticular hydrocarbons (CHCs) may provide reliable information for a parasitoid. However, the chemistry of CHC profiles of hosts living in processed stored food products is largely unknown. Here we showed that the ectoparasitoid Holepyris sylvanidis uses CHCs of its host Tribolium confusum, a worldwide stored product pest, as kairomones for host location and recognition at short range. Chemical analysis of T. confusum larval extracts by gas chromatography coupled with mass spectrometry revealed a rich blend of long-chain (C25-C30) hydrocarbons, including n-alkanes, mono-, and dimethylalkanes. We further studied whether host larvae leave sufficient CHCs on a substrate where they walk along, thus allowing parasitoids to perceive a CHC trail and follow it to their host larvae. We detected 18 CHCs on a substrate that had been exposed to host larvae. These compounds were also found in crude extracts of host larvae and made up about a fifth of the CHC amount extracted. Behavioral assays showed that trails of host CHCs were followed by the parasitoids and reduced their searching time until successful host recognition. Host CHC trails deposited on different substrates were persistent for about a day. Hence, the parasitoid H. sylvanidis exploits CHCs of T. confusum larvae for host finding by following host CHC trails and for host recognition by direct contact with host larvae.




Similar content being viewed by others
References
Afsheen S, Wang X, Li R, Zhu C-S, Lou Y-G (2008) Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci 15:381–397
Agelopoulos NG, Dicke M, Posthumus MA (1995) Role of volatile inforchemicals emitted by feces of larvae in host-searching behavior of parasitoid Cotesia rubecula (hymenoptera: Braconidae): a behavioral and chemical study. J Chem Ecol 21:1789–1811
Amante M, Schöller M, Hardy ICW, Russo A (2017a) Reproductive biology of Holepyris sylvanidis (hymenoptera: Bethylidae). Biol Control 106:1–8
Amante M, Schöller M, Suma P, Russo A (2017b) Bethylids attacking stored-product pests: an overview. Entomol Exp Appl 163:251–264
Athanassiou CG, Kavallieratos NG, Trematerra P (2006) Responses of Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae) to traps baited with pheromones and food volatiles. Eur J Entomol 103:371–378
Baker JE, Sukkestad DR, Woo SM, Nelson DR (1978) Cuticular hydrocarbons of Tribolium castaneum: effects of the food additive tricalcium phosphate. Insect Biochem 8:159–167
Borges M, Colazza S, Ramirez-Lucas P, Chauhan KR, Blassioli-Moraes MC, Aldrich JR (2003) Kairomonal effect of walking traces from Euschistus heros (Heteroptera: Pentatomidae) on two strains of Telenomus podisi (hymenoptera: Scelionidae). Physiol Entomol 28:349–355
Colazza S, Aquila G, De Pasquale C, Peri E, Millar JG (2007) The egg parasitoid Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J Chem Ecol 33:1405–1420
Colazza S, Lo Bue M, Lo Giudice D, Peri E (2009) The response of Trissolcus basalis to footprint contact kairomones from Nezara viridula females is mediated by leaf epicuticular waxes. Naturwissenschaften 96:975–981
Colazza S, Cusumano A, Lo Giudice D, Peri E (2014) Chemo-orientation responses in hymenopteran parasitoids induced by substrate-borne semiochemicals. BioControl 59:1–17
Collatz J, Steidle JLM (2008) Hunting for moving hosts: Cephalonomia tarsalis, a parasitoid of free-living grain beetles. Basic Appl Ecol 9:452–457
Collatz J, Fuhrmann A, Selzer P, Oehme RM, Hartelt K, Kimmig P, Meiners T, Mackenstedt U, Steidle JLM (2010) Being a parasitoid of parasites: host finding in the tick wasp Ixodiphagus hookeri by odours from mammals. Entomol Exp Appl 134:131–137
Conti E, Colazza S (2012) Chemical ecology of egg parasitoids associated with true bugs. Psyche 2012:1–11
Conti E, Salerno G, Leombruni B, Frati F, Bin F (2010) Short-range allelochemicals from a plant-herbivore association: a singular case of oviposition-induced synomone for an egg parasitoid. J Exp Biol 213:3911–3919
Dippel C, Hilker M (1998) Effects of physical and chemical signals on host foraging behavior of Drino inconspicua (Diptera: Tachinidae), a generalist parasitoid. Environ Entomol 27:682–687
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Evans HE (1977) A revision of the genus Holepyris in the Americas (hymenoptera: Bethylidae). Trans Am Entomol Soc 103:531–579
Fürstenau B, Adler C, Schulz H, Hilker M (2016) Host habitat volatiles enhance the olfactory response of the larval parasitoid Holepyris sylvanidis to specifically host-associated cues. Chem Senses 41:611–621
Gerhardt H, Betz O, Albert K, Lammerhofer M (2016) Insect adhesion secretions: similarities and dissimilarities in hydrocarbon profiles of tarsi and corresponding tibiae. J Chem Ecol 42:725–738
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
González JM, Cusumano A, Williams HJ, Colazza S, Vinson SB (2011) Behavioral and chemical investigations of contact kairomones released by the mud dauber wasp Trypoxylon politum, a host of the parasitoid Melittobia digitata. J Chem Ecol 37:629–639
Hebanowska E, Malinski E, Dubis E, Pihlaja K, Oksman P, Wiinamäki K, Nawrot J, Szafranek J (1989) The composition of cuticular hydrocarbons of the Tribolium destructor. Comp Biochem Physiol B Comp Biochem 93:437–442
Hebanowska E, Malinski E, Latowska A, Dubis E, Pihlaja K, Oksman P, Nawrot J, Szafranek J (1990) A comparison of cuticular hydrocarbons of larvae and beetles of the Tribolium destructor. Comp Biochem Physiol B Comp Biochem 96:815–819
Hilker M, McNeil J (2008) Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odorous environment. In: Wajnberg E, Bernstein C, van Alphen J (eds) Behavioural ecology of insect parasitoids. From theoretical approaches to field applications. Blackwell Publishing Ltd., Oxford, pp 693–705
Howard RW (1992) Comparative analysis of cuticular hydrocarbons from the ectoparasitoids Cephalonomia waterstoni and Laelius utilis (hymenoptera: Bethylidae) and their respective hosts, Cryptolestes ferrugineus (Coleoptera: Cucujidae) and Trogoderma variabile (Coleoptera: Dermestidae). Ann Entomol Soc Am 85:317–325
Howard RW (2001) Cuticular hydrocarbons of adult Pteromalus cerealellae (hymenoptera: Pteromalidae) and two larval hosts, Angoumois grain moth (Lepidoptera: Gelechiidae) and cowpea weevil (Coleptera: Bruchidae). Ann Entomol Soc Am 94:152–158
Howard RW, Flinn PW (1990) Larval trails of Cryptolestes ferrugineus (Coleoptera: Cucujidae) as kairomonal host-finding cues for the parasitoid Cephalonomia waterstoni (hymenoptera: Bethylidae). Ann Entomol Soc Am 83:239–245
Howard RW, Charlton M, Charlton RE (1998) Host-finding, host-recognition, and host-acceptance behavior of Cephalonomia tarsalis (hymenoptera: Bethylidae). Ann Entomol Soc Am 91:879–889
Iacovone A et al (2016) The role of contact chemoreception in the host location process of an egg parasitoid. J Insect Physiol 91-92:63–75
IBM Corp. Released (2013) IBM SPSS Statistics for Windows, Version 22.0. IBM Corp: Armonk, NY
Lo Giudice D, Riedel M, Rostás M, Peri E, Colazza S (2011) Host sex discrimination by an egg parasitoid on brassica leaves. J Chem Ecol 37:622–628
Lockey KH (1978) Hydrocarbons of adult Tribolium castaneum Hbst. And Tribolium confusum Duv. (Coleoptera: Tenebrionidae). Comp Biochem Physiol B Comp Biochem 61:401–407
Mathis KA, Tsutsui ND (2016) Cuticular hydrocarbon cues are used for host acceptance by Pseudacteon spp. Phorid flies that attack Azteca sericeasur ants. J Chem Ecol 42:286–293
Mattiacci L, Dicke M (1995) The parasitoid Cotesia glomerata (hymenoptera: Braconidae) discriminates between first and fifth larval instars of its host Pieris brassicae, on the basis of contact cues from frass, silk, and herbivore-damaged leaf tissue. J Insect Behav 8:485–498
Morgan ED (2009) Trail pheromones of ants. Physiol Entomol 34:1–17
Park T (1934) Observations on the general biology of the flour beetle, Tribolium confusum. Q Rev Biol 9:36–54
Peri E, Frati F, Salerno G, Conti E, Colazza S (2013) Host chemical footprints induce host sex discrimination ability in egg parasitoids. PLoS One 8:e79054
Peri E, Salerno G, Slimani T, Frati F, Conti E, Colazza S, Cusumano A (2016) The response of an egg parasitoid to substrate-borne semiochemicals is affected by previous experience. Sci Rep 6:27098
Quicke DLJ (1997) Parasitic wasps. Chapman & Hall Ltd, New York
R Core Team (2014) R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing
Ren C, Webster P, Finkel SE, Tower J (2007) Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab 6:144–152
Rostás M, Wölfling M (2009) Caterpillar footprints as host location kairomones for Cotesia marginiventris: persistence and chemical nature. J Chem Ecol 35:20–27
Rostás M, Ruf D, Zabka V, Hildebrandt U (2008) Plant surface wax affects parasitoid's response to host footprints. Naturwissenschaften 95:997–1002
Rutledge CE (1996) A survey of identified kairomones and synomones used by insect parasitoids to locate and accept their hosts. Chemoecology 131:121–131
Salerno G, Frati F, Conti E, De Pasquale C, Peri E, Colazza S (2009) A finely tuned strategy adopted by an egg parasitoid to exploit chemical traces from host adults. J Exp Biol 212:1825–1831
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci 107:20051–20056
Steidle JLM, Van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol Exp Appl 108:133–148
Suzuki T, Nakakita H, Kuwahara Y (1988) Defensive secretions and hydrocarbons of two Tribolium species and their hybrids (Coleoptera: Tenebrionidae). Appl Entomol Zool 23:329–337
Thibout E (2005) Role of caterpillar silk thread in location of host pupae by the parasitoid Diadromus pulchellus. J Insect Behav 18:817–826
Van Alphen JJM, Jervis MA (1996) Foraging behaviour. In: Jervis MA, Kidd N (eds) Insect natural enemies: practical approaches to their study and evaluation. Springer Netherlands, Dordrecht, pp 1–62
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463–471
Vayias BJ, Athanassiou CG, Buchelos CT (2006) Evaluation of three diatomaceous earth and one natural pyrethrum formulations against pupae of Tribolium confusum DuVal (Coleoptera: Tenebrionidae) on wheat and flour. Crop Prot 25:766–772
Vinson SB (1998) The general host selection behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96
Wajnberg E, Colazza S (2013) Chemical ecology of parasitoids. Wiley-Blackwell, Chichester
Wajnberg E, Bernstein C, van Alphen J (2008) Behavioural ecology of insect parasitoids. From theoretical approaches to field applications. Blackwell Publishing Ltd., Oxford
Acknowledgements
We gratefully acknowledge the help by Heidrun Anders (Institute for Ecological Chemistry, Plant Analysis and Stored Product Protection, Julius Kühn-Institut, Berlin, Germany) for help and advice in rearing T. confusum and H. sylvanidis specimens.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 2186 kb)
Rights and permissions
About this article
Cite this article
Fürstenau, B., Hilker, M. Cuticular Hydrocarbons of Tribolium confusum Larvae Mediate Trail Following and Host Recognition in the Ectoparasitoid Holepyris sylvanidis . J Chem Ecol 43, 858–868 (2017). https://doi.org/10.1007/s10886-017-0885-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0885-1