Abstract
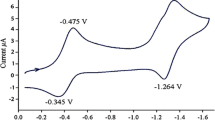
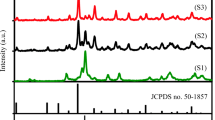
Single crystals of bis(3-aminopyridinium)tetrachlorocobaltate(II) were successfully synthesized and structurally characterized using single crystal X-ray diffraction. This study shows that the structure is built on inorganic anionic and organic cationic subnetworks stabilized by Cl…Cl and antiparallel offset face to face π-π stacking interactions, respectively. The cohesion of the overall packing is ensured by N–H…Cl hydrogen bonds leading to a three dimensional network. Structural geometry optimization and gap energy determination were performed thanks to DFT calculation. Thermal decomposition of the title compound powder, at 450 °C in air atmosphere, leads to nanostructured Co3O4 as confirmed by X-ray powder diffraction. The obtained cobalt oxide was used as adsorbent for the removal of hexavalent chromium ions from aqueous solution. The effect of contact time was investigated and showed an unusual behavior consisting of the existence of two equilibrium plateaus at 11 mg.g−1 and 15 mg.g−1 which were attributed to surface heterogeneity, as elucidated by scanning electron microscopy analysis. As a consequence, the kinetic data were analyzed in steps and fitted using non linear pseudo-first order and non linear pseudo-second order models. A comparative study with other adsorbents demonstrated the potential of the synthesized Co3O4 via thermal decomposition of the title compound for hexavalent chromium ions removal.









Similar content being viewed by others
References
S. Upreti and A. Ramanan (2006). Cryst. Growth Des. 6 (9), 2066–2071. https://doi.org/10.1021/cg0601610.
C. B. Aakeröy and D. S. Leinen, Hydrogen-bond assisted assembly of organic and organic-inorganic solids,. Crystal Engineering: From Molecules and Crystals to Materials. Springer, Dordrecht, pp 89–106.
J. Zhao, F. Chen, Y. Han, H. Chen, Z. Luo, H. Tian, Y. Zhao, A. Ma, and L. Zhu (2018). Molecules 23 (6), 1397–1410. https://doi.org/10.3390/molecules23061397.
M. L. Nisbet, Y. Wang, and K. R. Poeppelmeier (2020). Cryst. Growth Des. 21 (1), 552–562. https://doi.org/10.1021/acs.cgd.0c01355.
D. J. Carnevale, C. P. Landee, M. M. Turnbull, M. Winn, and F. Xiao (2010). J. Coord. Chem. 63 (13), 2223–2238. https://doi.org/10.1080/00958972.2010.502230.
J. M. Land, R. G. Baughman, and C. A. Hester (1997). Acta Cryst. https://doi.org/10.1107/S0108270197099435.
S. Haddad, A. Vij, and R. D. Willett (2003). J. Chem. Crystallogr. 33 (4), 245–251. https://doi.org/10.1023/A:1023872925437.
S. R. Jebas, T. Balasubramanian, and M. E. Light (2006). Acta Cryst. E62 (8), m1818–m1819. https://doi.org/10.1107/S1600536806026213.
S. R. Jebas, A. Sinthiya, B. Ravindran Durai Nayagam, D. Schollmeyer, and S. A. C. Raj (2009). Acta Cryst. https://doi.org/10.1107/S1600536809013270.
M. Mghandef and H. Boughzala (2015). Acta Cryst. E71 (5), 555–557. https://doi.org/10.1107/S2056989015007707.
O. Ben Moussa, H. Chebbi, and M. F. Zid (2018). Acta Cryst. https://doi.org/10.1107/S2056989018003171.
A. A. Alotaibi, C. Ayari, E. Bajuavfir, A. Ahmad, F. Al-Nahdi, A. M. Alswieleh, K. L. Alotaibi, J. Mi, C. Ben Nasr, and M. H. Mrad (2022). Opt. Spectrosc. Thermal Anal. Cryst. 12 (2), 140–157. https://doi.org/10.3390/cryst12020140.
Y. L. Xu, J. Zhang, L. S. Chen, Y. Y. Zeng, J. R. Zhou, C. L. Ni, and W. X. Zheng (2020). J. Mol. Struct. 1222, 128902–128907. https://doi.org/10.1016/j.molstruc.2020.128902.
A. Kaiba, M. H. Geesi, Y. Riadi, E. O. Ibnouf, T. A. Aljohani, and P. Guionneau (2021). J Solid State Chem. https://doi.org/10.1016/j.jssc.2021.122587.
A. Timoumi, D. Dastan, B. Jamoussi, K. Essalah, O. Alsalmi, N. Bouguila, H. Abassi, R. Chakroun, Z. Shi, and Ş Ţălu (2022). Mol. 27 (19), 6151. https://doi.org/10.3390/molecules27196151.
S. Hadaoui, Z. Ouerghi, S. Elleuch, and R. Kefi (2022). J Struct Mol. https://doi.org/10.1016/j.molstruc.2021.131441.
Z. Abbasi, M. Salehi, A. Khaleghian, and M. Kubicki (2018). Appl. Organomet. Chem. 32 (11), e4542. https://doi.org/10.1002/aoc.4542.
E. M. M. Ibrahim, L. H. Abdel-Rahman, A. M. Abu-Dief, A. Elshafaie, S. K. Hamdan, and A. M. Ahmed (2018). Mater. Res. Bull. 99, 103–108. https://doi.org/10.1016/j.materresbull.2017.11.002.
B. Z. Momeni, F. Rahimi, and F. Rominger (2018). J. Inorg. Organomet. Polym. Mater. 28 (1), 235–250. https://doi.org/10.1007/s10904-017-0706-6.
Z. Razmara and E. Sanchooli (2019). J. Inorg. Organomet. Polym. Mater. 29 (6), 2090–2102. https://doi.org/10.1007/s10904-019-01168-2.
S. Meghdadi, M. Amirnasr, M. Zhiani, F. Jallili, M. Jari, and M. Kiani (2017). Electrocatalysis 8 (2), 122–131. https://doi.org/10.1007/s12678-016-0345-7.
G. M. Sheldrick (2008). Acta Cryst. A 64, 112–122.
R. Gara, M. O. Zouaghi, L. M. H. Alshandoudi, and Y. Arfaoui (2021). J. Mol. Model. 27 (5), 1–12. https://doi.org/10.1007/s00894-021-04729-w.
Gaussian 09, Revision A.1, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch (1994). J. Phys. Chem. 98 (45), 11623–11627. https://doi.org/10.1021/j100096a001.
R. B. J. S. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople (1980). J. Chem. Phys. 72 (1), 650–654. https://doi.org/10.1063/1.438955.
A. Wang, Y. Wang, J. Jia, L. Feng, C. Zhang, and L. Liu (2013). J. Phys. Chem. A. 117, 5061–5072. https://doi.org/10.1021/jp403145h.
S. F. Lo, S. Y. Wang, M. J. Tsai, and L. D. Lin (2020). Chem. Eng. Res. Design 90 (9), 1397–1406. https://doi.org/10.1016/j.cherd.2011.11.020.
L. Yang, D. R. Powell, and R. P. Houser (2007). Dalton Trans. 9, 955–964. https://doi.org/10.1039/B617136B.
I. Chérif, M. F. Zid, M. El-Ghozzi, and D. Avignant (2012). Acta Cryst. https://doi.org/10.1107/S1600536812025020.
M. Chao, E. Schemp, and R. D. Rosenstein (1975). Acta Cryst. B31 (12), 2924–2926. https://doi.org/10.1107/S0567740875009284.
S. Dgachi, M. M. Turnbull, F. Mezzadri, A. J. Norquist, A. Soran, J. Boonmak, G. Nemes, and H. Naïli (2021). Inorganica Chim Acta. https://doi.org/10.1016/j.ica.2020.119997.
S. Dgachi, F. Rahmouni, A. Soran, M. Saoudi, G. Nemes, and H. Naïli (2021). J Mol Struct. https://doi.org/10.1016/j.molstruc.2021.130996.
C. Orek, B. Gündüz, O. Kaygili, and N. Bulut (2017). Chem. Phys. Lett. 678, 130–138. https://doi.org/10.1016/j.cplett.2017.04.050.
N. M. Juibari, A. Abbasi, M. Najafi, and S. Geranmayeh (2015). C. R. Chimie 18, 662–667. https://doi.org/10.1016/j.crci.2014.11.006.
P. Raghunath, M. A. Reddy, C. Gouri, K. Bhanuprakash, and V. J. Rao (2006). J. Phys. Chem. A. 110, 1152–1162. https://doi.org/10.1021/jp0555753.
A. M. B. Salah, R. P. Herrera, and H. Naïli (2018). J. Mol. Struct. 1165, 356–362. https://doi.org/10.1016/j.molstruc.2018.04.002.
M. Rabiei, A. Palevicius, A. Monshi, S. Nasiri, A. Vilkauskas, and G. Janusas (2020). Nanomaterials 10 (9), 1627. https://doi.org/10.3390/nano10091627.
M. Bizi (2020). Molecules 25 (20), 4656. https://doi.org/10.3390/molecules25204656.
E. D. Revellame, D. L. Fortela, W. Sharp, R. Hernandez, and M. E. Zappi (2020). Cleaner Eng Technol. https://doi.org/10.1016/j.clet.2020.100032.
L. J. Martínez, A. Muñoz-Bonilla, E. Mazario, F. J. Recio, F. J. Palomares, and P. Herrasti (2015). Int. J. Environ. Sci. Technol. 12, 4017–4024. https://doi.org/10.1007/s13762-015-0832-z.
V. K. Gupta, R. Chandra, I. Tyagi, and M. Verma (2016). J. Colloid Interface Sci. 478, 54–62. https://doi.org/10.1016/j.jcis.2016.05.064.
S. K. Ashan, N. Ziaeifara, and M. Khosravi (2016). Orient J Chem 32 (1), 749–758. https://doi.org/10.13005/ojc/320184.
Acknowledgements
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Contributions
IC: Conceptualization – single crystal structure determination – formal analysis—writing original draft—editing. SH, IJ and YA: Theoretical calculation – writing review. FM: Adsorption experiments—formal analysis. DD: Writing review—editing. MF: Morphological investigation – writing review—editing. MFZ: Single crystal X-ray diffraction data collection – discussion. SA: Discussion – writing review.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chérif, I., Hassen, S., Jendoubi, I. et al. A New Hybrid Organic–Inorganic Salt: bis(3-Aminopyridinium)Tetrachlorocobaltate(II), Application in the Synthesis of Nanostructured Co3O4 for Hexavalent Chromium Removal. J Clust Sci 34, 3047–3059 (2023). https://doi.org/10.1007/s10876-023-02446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02446-3