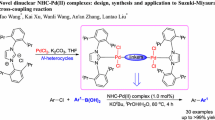
Abstract
A series of N-heterocyclic carbene (NHC)–palladium catalysts have been synthesized and applied to catalyze the Suzuki coupling reaction efficiently between aryl sulfonates and arylboronic acids with the potassium phosphate heptahydrate as a base. The desired yields are obtained even with less reactive aryl tosylates or aryl mesylates as substrates. This method was applied successfully to the synthesis of the (R)-2-(t-butoxycarbonylamino)-3-(biphenyl-4-yl)-propan-1-ol which is the key intermediate of sacubitril, a component of the newly approved antihypertensive drug “Entresto.”
Graphic abstract





Similar content being viewed by others
References
Miyaura N, Yamada K, Suzuki A (1979) A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett 20:3437–3440. https://doi.org/10.1016/S0040-4039(01)95429-2
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95:2457–2483. https://doi.org/10.1021/cr00039a007
Suzuki A (1999) Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J Organomet Chem 576:147–168. https://doi.org/10.1016/S0022-328X(98)01055-9
Miyaura N (2002) Cross-coupling reaction of organoboron compounds via base-assisted transmetalation to palladium (II) complexes. J Organomet Chem 653:54–57. https://doi.org/10.1016/S0022-328X(02)01264-0
Suzuki A (2005) Carbon–carbon bonding made easy. Chem Commun 38:4759–4763. https://doi.org/10.1039/B507375H
Alonso F, Yus M, Beletskaya IP (2008) Non-conventional methodologies for transition-metal catalysed carbon–carbon coupling: a critical overview. Part 2: the Suzuki reaction. Tetrahedron 64:3047–3101. https://doi.org/10.1016/j.tet.2007.12.036
Molander GA, Canturk B (2009) Organotrifluoroborates and monocoordinated palladium complexes as catalysts—a perfect combination for Suzuki–Miyaura coupling. Angew Chem Int Edit 48:9240–9261. https://doi.org/10.1002/anie.200904306
Ackermann L, Potukuchi HK, Althammer A, Born R, Mayer P (2010) Tetra-ortho-substituted biaryls through palladium-catalyzed Suzuki–Miyaura couplings with a diaminochlorophosphine ligand. Org Lett 12:1004–1007. https://doi.org/10.1021/ol1000186
Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M (2002) Aryl–aryl bond formation one century after the discovery of the Ullmann reaction. Chem Rev 102:1359–1470. https://doi.org/10.1021/cr000664r
Corbet JP, Mignani G (2006) Selected patented cross-coupling reaction technologies. Chem Rev 106:2651–2710. https://doi.org/10.1021/cr0505268
Martin R, Buchwald SL (2008) Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc Chem Res 41:1461–1473. https://doi.org/10.1021/ar800036s
Wu XF, Anbarasan P, Neumann H, Beller M (2010) From noble metal to Nobel prize: palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew Chem Int Edit 49:9047–9050. https://doi.org/10.1002/anie.201006374
Limmert ME, Roy AH, Hartwig JF (2005) Kumada coupling of aryl and vinyl tosylates under mild conditions. J Org Chem 70:9364–9370. https://doi.org/10.1021/jo051394l
Wilson DA, Wilson CJ, Moldoveanu C, Resmerita AM, Corcoran P, Hoang LM, Percec V (2010) Neopentylglycolborylation of aryl mesylates and tosylates catalyzed by Ni-based mixed-ligand systems activated with Zn. J Am Chem Soc 132:1800–1801. https://doi.org/10.1021/ja910808x
Gao CY, Yang LM (2008) Nickel-catalyzed amination of aryl tosylates. J Org Chem 73:1624–1627. https://doi.org/10.1021/jo7022558
Ackermann L, Althammer A (2006) Air-stable PinP (O) H as preligand for palladium-catalyzed Kumada couplings of unactivated tosylates. Org Lett 8:3457–3460. https://doi.org/10.1021/ol061116o
Zim D, Lando VR, Dupont J, Monteiro AL (2001) NiCl2 (PCy3) 2: a simple and efficient catalyst precursor for the Suzuki cross-coupling of aryl tosylates and arylboronic acids. Org Lett 3:3049–3051. https://doi.org/10.1021/ol016526l
Roy AH, Hartwig JF (2003) Oxidative addition of aryl tosylates to palladium (0) and coupling of unactivated aryl tosylates at room temperature. J Am Chem Soc 125:8704–8705. https://doi.org/10.1021/ja035835z
Shieh WC, Carlson JA (1992) A simple asymmetric synthesis of 4-arylphenylalanines via palladium-catalyzed cross coupling reaction of arylboronic acids with tyrosine triflate. J Org Chem 57:379–381. https://doi.org/10.1021/jo00027a066
Wang ZY, Chen GQ, Shao LX (2012) N-heterocyclic carbene–palladium (II)–1-methylimidazole complex-catalyzed Suzuki–Miyaura coupling of aryl sulfonates with arylboronic acids. J Org Chem 77:6608–6614. https://doi.org/10.1021/jo301270t
Tang Y, Yang F, Nie S, Wang L, Luo Z, Lu H (2015) Synthesis of a carbene complex and its application in Suzuki reaction. Chin J Org Chem 35:705–711. https://doi.org/10.6023/cjoc201408028
O’Brien CJ, Kantchev EAB, Valente C, Hadei N, Chass GA, Lough A, Organ MG (2006) Easily prepared air-and moisture-stable Pd–NHC (NHC=N-heterocyclic carbene) complexes: a reliable, user-friendly, highly active palladium precatalyst for the Suzuki–Miyaura reaction. Chem Eur J 12:4743–4748. https://doi.org/10.1002/chem.200600251
Viciu MS, Grasa GA, Nolan SP (2001) Catalytic dehalogenation of aryl halides mediated by a palladium/imidazolium salt system. Organometallics 20:3607–3612. https://doi.org/10.1021/om010332s
Palenik RC, Palenik GJ (1992) Aqueous organometallic chemistry. Synthesis of bis(n3-ALLYL)-μ-dichlorodipalladium(II). Synth React Inorg M 22:1395–1399. https://doi.org/10.1080/15533179208017850
Viciu MS, Kissling RM, Stevens ED, Nolan SP (2002) An air-stable palladium/N-heterocyclic carbene complex and its reactivity in aryl amination. Org Lett 4:2229–2231. https://doi.org/10.1021/ol0260831
Navarro O, Kelly RA, Nolan SP (2003) A general method for the Suzuki–Miyaura cross-coupling of sterically hindered aryl chlorides: synthesis of di- and tri-ortho-substituted biaryls in 2-propanol at room temperature. J Am Chem Soc 125:16194–16195. https://doi.org/10.1021/ja038631r
Viciu MS, Kelly RA, Stevens ED, Naud F, Studer M, Nolan SP (2003) Synthesis, characterization, and catalytic activity of N-heterocyclic carbene (NHC) palladacycle complexes. Org Lett 5:1479–1482. https://doi.org/10.1021/ol034264c
Trost BM, Weber L, Strege PE, Fullerton TJ, Dietsche TJ (1978) Allylic alkylation: nucleophilic attack on. pi.-allylpalladium complexes. J Am Chem Soc 100:3416–3426. https://doi.org/10.1021/ja00479a025
Bastug G, Nolan SP (2013) Carbon–sulfur bond formation catalyzed by [Pd (IPr*OMe)(cin) Cl](Cin = cinnamyl). J Org Chem 78:9303–9308. https://doi.org/10.1021/jo401492n
Navarro O, Kaur H, Mahjoor P, Nolan SP (2004) Cross-coupling and dehalogenation reactions catalyzed by (N-heterocyclic carbene) Pd(allyl)Cl complexes. J Org Chem 69:3173–3180. https://doi.org/10.1021/jo035834p
Chartoire A, Lesieur M, Falivene L, Slawin AM, Cavallo L, Cazin CS, Nolan SP (2012) [Pd(IPr*)(cinnamyl) Cl]: an efficient pre-catalyst for the preparation of tetra-ortho-substituted biaryls by Suzuki–Miyaura cross-coupling. Chem Eur J 18:4517–4521. https://doi.org/10.1002/chem.201104009
Dröge T, Glorius F (2010) The measure of all rings—N-heterocyclic carbenes. Angew Chem Int Edit 49:6940–6952. https://doi.org/10.1002/anie.201001865
Hahn FE, Jahnke MC (2008) Heterocyclic carbenes: synthesis and coordination chemistry. Angew Chem Int Edit 47:3122–3172. https://doi.org/10.1002/anie.200703883
Fortman GC, Nolan SP (2011) N-heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem Soc Rev 40:5151–5169. https://doi.org/10.1039/C1CS15088J
Diez-Gonzalez S, Marion N, Nolan SP (2009) N-heterocyclic carbenes in late transition metal catalysis. Chem Rev 109:3612–3676. https://doi.org/10.1021/cr900074m
Gallop CW (2015) N-heterocyclic carbene-palladium and-copper complexes in cross-coupling reactions (Doctoral dissertation, University of Sussex)
Takeda Y, Ikeda Y, Kuroda A, Tanaka S, Minakata S (2014) Pd/NHC-catalyzed enantiospecific and regioselective Suzuki–Miyaura arylation of 2-arylaziridines: synthesis of enantioenriched 2-arylphenethylamine derivatives. J Am Chem Soc 136:8544–8547. https://doi.org/10.1021/ja5039616
Yadav MR, Nagaoka M, Kashihara M, Zhong RL, Miyazaki T, Sakaki S, Nakao Y (2017) The Suzuki–Miyaura coupling of nitroarenes. J Am Chem Soc 139:9423–9426. https://doi.org/10.1021/jacs.7b03159
Acknowledgements
We gratefully acknowledge the Science and Technology Department of Sichuan Province (2019JDJQ0010). We would also like to thank Dr. Jinkun Huang for helpful comments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Q., Dai, Z., Di, X. et al. Pd(NHC)(cinnamyl)Cl-catalyzed Suzuki cross-coupling reaction of aryl sulfonates with arylboronic acids. Mol Divers 24, 903–911 (2020). https://doi.org/10.1007/s11030-019-10001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-10001-4