Abstract
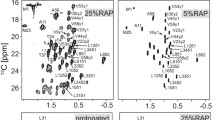
We present a systematic study of the effect of the level of exchangeable protons on the observed amide proton linewidth obtained in perdeuterated proteins. Decreasing the amount of D2O employed in the crystallization buffer from 90 to 0%, we observe a fourfold increase in linewidth for both 1H and 15N resonances. At the same time, we find a gradual increase in the signal-to-noise ratio (SNR) for 1H–15N correlations in dipolar coupling based experiments for H2O concentrations of up to 40%. Beyond 40%, a significant reduction in SNR is observed. Scalar-coupling based 1H–15N correlation experiments yield a nearly constant SNR for samples prepared with ≤30% H2O. Samples in which more H2O is employed for crystallization show a significantly reduced NMR intensity. Calculation of the SNR by taking into account the reduction in 1H T 1 in samples containing more protons (SNR per unit time), yields a maximum SNR for samples crystallized using 30 and 40% H2O for scalar and dipolar coupling based experiments, respectively. A sensitivity gain of 3.8 is obtained by increasing the H2O concentration from 10 to 40% in the CP based experiment, whereas the linewidth only becomes 1.5 times broader. In general, we find that CP is more favorable compared to INEPT based transfer when the number of possible 1H,1H interactions increases. At low levels of deuteration (≥60% H2O in the crystallization buffer), resonances from rigid residues are broadened beyond detection. All experiments are carried out at MAS frequency of 24 kHz employing perdeuterated samples of the chicken α-spectrin SH3 domain.





Similar content being viewed by others
References
Chevelkov V, Reif B (2008) TROSY effects in MAS solid-state NMR. Concepts Magn Reson Part B 32:143–156
Chevelkov V, Rossum BJ, Castellani F, Rehbein K, Diehl A, Hohwy M, Steuernagel S, Engelke F, Oschkinat H, Reif B (2003) 1H detection in MAS solid-state NMR spectroscopy of biomacromolecules employing pulsed field gradients for residual solvent suppression. J Am Chem Soc 125:7788–7789
Chevelkov V, Rehbein K, Diehl A, Reif B (2006) Ultrahigh resolution in proton solid-state NMR spectroscopy at high levels of deuteration. Angew Chem Int Ed 45:3878–3881
Chevelkov V, Diehl A, Reif B (2008) Measurement of 15N–T1 relaxation rates in a perdeuterated protein by magic angle spinning solid-state nuclear magnetic resonance spectroscopy. J Chem Phys 128:052316
Ishii Y, Tycko R (2000) Sensitivity enhancement in solid state 15N NMR by indirect detection with high-speed magic angle spinning. J Magn Reson 142:199–204
Ishii Y, Yesinowski JP, Tycko R (2001) Sensitivity enhancement in solid-state 13C NMR of synthetic polymers and biopolymers by 1H NMR detection with high-speed magic angle spinning. J Am Chem Soc 123:2921–2922
Laage S, Sachleben JR, Steuernagel S, Pierattelli R, Pintacuda G, Emsley L (2009) Fast acquisition of multi-dimensional spectra in solid-state NMR enabled by ultra-fast MAS. J Magn Reson 196:133–141
Leskes M, Madhu PK, Vega S (2007) A broad-band z-rotation windowed phase-modulated Lee-Goldburg pulse sequence for 1H spectroscopy in solid-state NMR. Chem Phys Lett 447:370–374
Linser RJ, Chevelkov V, Diehl A, Reif B (2007) Sensitivity enhancement using paramagnetic relaxation in MAS solid-state NMR of perdeuterated proteins. J Magn Reson 189:209–216
Linser R, Fink U, Reif B (2008) Proton-detected scalar coupling based assignment strategies in MAS solid-state NMR spectroscopy applied to perdeuterated proteins. J Magn Reson 193:89–93
Morcombe CR, Paulson EK, Gaponenko V, Byrd A, Zilm KW (2005) 1H-5N correlation spectroscopy of nanocrystalline proteins. J Bio NMR 31:217–230
Paulson EK, Morcombe CR, Gaponenko V, Dancheck B, Byrd RA, Zilm KW (2003) Sensitive high resolution inverse detection NMR spectroscopy of proteins in the solid state. J Am Chem Soc 125:15831–15836
Rossum BJ, Castellani F, Pauli J, Rehbein K, Hollander J, de Groot HJM, Oschkinat H (2003) Assignment of amide proton signals by combined evaluation of HN, NN and HNCA MAS–NMR correlation spectra. J Biomol NMR 25:217–223
Salager E, Stein RS, Steurnagel S, Lesage A, Elena B, Emsley L (2009) Enhanced sensitivity in high-resolution 1H solid-state NMR spectroscopy with DUMBO dipolar decoupling under ultra-fast MAS. Chem Phys Lett 469:336–341
Vinogradov E, Madhu PK, Vega S (1999) High-resolution proton solid-state NMR spectroscopy by phase-modulated Lee-Goldburg experiment. Chem Phys Lett 314:443–450
Wickramasinghe NP, Ishii Y (2007) Sensitivity enhancement in 13C solid-state NMR of protein microcrystals by use of paramagnetic metal ion for optimizing 1H T1 relaxation. J Magn Reson 184:350–356
Zhou DH, Rienstra CM (2008) Rapid analysis of organic compounds by proton-detected heteronuclear correlation NMR spectroscopy with 40 kHz magic-angle spinning. Angew Chem 47:7328–7331
Zhou DH, Graesser DT, Franks WT, Rienstra CM (2006) Sensitivity and resolution in proton solid-state NMR at intermediate deuteration levels: quantitative linewidth characterization and applications to correlation spectroscopy. J Magn Reson 178:297–307
Zhou DH, Shah G, Cormos M, Mullen C, Sandoz D, Rienstra CM (2007) Proton-detected solid-state NMR spectroscopy of fully protonated proteins at 40 kHz magic-angle spinning. J Am Chem Soc 129:11791–11801
Author information
Authors and Affiliations
Corresponding author
Additional information
Ümit Akbey and Sascha Lange contributed equally.
Rights and permissions
About this article
Cite this article
Akbey, Ü., Lange, S., Trent Franks, W. et al. Optimum levels of exchangeable protons in perdeuterated proteins for proton detection in MAS solid-state NMR spectroscopy. J Biomol NMR 46, 67–73 (2010). https://doi.org/10.1007/s10858-009-9369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-009-9369-0