Abstract
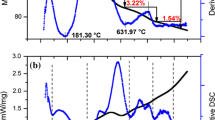
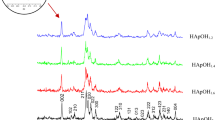
This paper focus on physicochemical changes in bio-hydroxyapatite (BIO-HAp) from bovine femur obtained by calcination at high temperatures: 520–620 (each 20 °C) at 7.4 °C/min and from 700 to 1100 °C (each 100 °C) at three heating rates: 7.4, 9.9, and 11.1 °C/min. BIO-HAp samples were obtained using a multi-step process: cleaning, milling, hydrothermal process, calcination in an air atmosphere, and cooling in furnace air. Inductively Couple Plasma (ICP) showed that the presence of Mg, K, S, Ba, Zn, and Na, is not affected by the annealing temperature and heating rate. While Scanning Electron Microscopy (SEM) images showed the continuous growth of the HAp crystals during the calcination process due to the coalescence phenomenon, and the Full Width at the Half Maximum for the X-ray patterns for temperatures up to 700 is affected by the annealing temperature and the heating rate. Through X-ray diffraction, thermal, and calorimetric analysis (TGA-DSC), a partial dehydroxylation of hydroxyapatite was found in samples calcined up to 900 °C for the three heating rates. Also, Ca/P molar ratio decreased for samples calcined up to 900 °C as a result of the dehydroxylation process. NaCaPO4, CaCO3, Ca3(PO4)2, MgO, and Ca(H2PO4)2 are some phases identified by X-ray diffraction; some of them are part of the bone and others were formed during the calcination process as a function of annealing temperature and heating rate, as it is the case for MgO.









Similar content being viewed by others
References
Londoño-Restrepo SM, Ramirez-Gutierrez CF, del Real A, Rubio-Rosas E, Rodriguez-García ME. Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J Mater Sci. 2016;51(9):4431–41.
Sofronia AM, Baies R, Anghel EM, Marinescu CA, Tanasesc S. Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater Sci Eng C. 2014;43:153–63.
Vallet-Regí M, González-Calbet JM. Calcium phosphates as substitution of bone tissues. Prog Solid State Chem. 2004;32(1):1–31.
Campa Molina J, Ulloa Godínez GS, Bucio Galindo L, Belío IA, Velazquez R, Rivera Muñoz EM. Chapter II, Tejido óseo y esmalte dental. In: Biomateriales: Fundamentos, técnicas y aplicaciones. Jalisco: Universidad de Guadalajara; 2007. p. 39–91.
Behari J Elements of bone biophysics. In: biophysical bone behavior: principles and application. Chichester: Wiley; 2009. p.1–52.
Barakat NA, Khil MS, Omran AM, Sheikh FA, Kim HY. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J Mater Process Technol. 2009;209(7):3408–15.
Sobczak-Kupiec A, Wzorek Z. The influence of calcination parameters on free calcium oxide content in natural hydroxyapatite. Cer Inter. 2012;38(1):641–7.
Nasiri-Tabrizi B, Fahami A, Ebrahimi-Kahrizsangi R. A comparative study of hydroxyapatite nanostructures produced under different milling conditions and thermal treatment of bovine bone. J Ind Eng Chem. 2014;20(1):245–58.
Li Z, Thompson BC, Dong Z, Khor KA. Optical and biological properties of transparent nanocrystalline hydroxyapatite obtained through spark plasma sintering. Mater Sci Eng C. 2016;69:956–66.
Eslami N, Mahmoodian R, Hamdi M, Khatir NM, Herliansyah MK, Rafieerad AR. Study the synthesis, characterization and immersion of dense and porous bovine hydroxyapatite structures in Hank’s balanced salt solution. JOM. 2017;69(4):691–8.
Ramirez-Gutierrez CF, Londoño-Restrepo SM, del Real A, Mondragón MA, Rodriguez-García ME. Effect of the temperature and sintering time on the thermal, structural, morphological, and vibrational properties of hydroxyapatite derived from pig bone. Cer Inter. 2017;43(10):7552–9.
Bahrololoom ME, Javidi M, Javadpour S, Ma J. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J Ceram Process Res. 2009;10:129–38.
Herliansyah MK, Hamdi M, Ide-Ektessabi A, Wildan MW, Toque JA. The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater Sci Eng C. 2009;29(5):1674–80.
Ooi CY, Hamdi M, Ramesh S. Properties of hydroxyapatite produced by annealing of bovine bone. Ceram Int. 2007;33(7):1171–7.
Ramirez-Gutierrez CF, Palechor-Ocampo AF, Londoño-Restrepo SM, Millán-Malo BM, Rodriguez-García ME. Cooling rate effects on thermal, structural, and microstructural properties of bio-hydroxyapatite obtained from bovine bone. J Biomed Res Part B. 2016;104(2):339–44.
Nasiri-Tabrizi B, Fahami A, Ebrahimi-Kahrizsangi R. Effect of milling parameters on the formation of nanocrystalline hydroxyapatite using different raw materials. Cer Int. 2013;39(5):5751–63.
Elliott JC. Structure and chemistry of the apatites and other calcium orthophosphates. 1st ed. Amsterdam: Elsevier; 1998.
Figueiredo M, Fernando A, Martins G, Freitas J, Judas F, Figueiredo H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram Int. 2010;36(8):2383–93.
Sobczak A, Kowalski Z, Wzorek Z. Preparation of hydroxyapatite from animal bones. Acta Bioeng Biomech. 2009;11(4):23–28.
Yoganand CP, Selvarajan V, Goudouri OM, Paraskevopoulos KM, Wu J, Xue D. Preparation of bovine hydroxyapatite by transferred arc plasma. Curr Appl Phys. 2011;11(3):702–9.
Giraldo-Betancur AL, Espinoza-Arbeláez DG, del Real-López A, Millán-Malo BM, Rivera-Muñoz E, Gutiérrez-Cortez E, Pineda-Gómez P, Jiménez-Sandoval SJ, Rodriguez-Garcia ME. Comparison of physicochemical properties of bio and commercial hydroxyapatite. Curr Appl Phys. 2013;13:1383–90.
Akram M, Ahmed R, Shakir I, Ibrahim WAW, Hussain R. Extracting hydroxyapatite and its precursors from natural resources. J Mater Sci. 2014;49(4):1461–75.
Joschek S, Nies B, Krotz R, Göpferich A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials. 2000;21(16):1645–58.
Tõnsuaadu K, Gross KA, Plūduma L, Veiderma M. A review on the thermal stability of calcium apatites. J Therm Anal Calorim. 2012;110(2):647–59.
Raynaud S, Champion E, Bernache-Assollant D, Thomas P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials. 2002;23(4):1065–72.
Ishikawa K, Ducheyne P, Radin S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J Mater Sci Mater Med. 1993;4(2):165–8.
Trombe JC, Montel G. Some features of the incorporation of oxygen in different oxidation states in the apatitic lattice—I On the existence of calcium and strontium oxyapatites. J Inorg Nucl Chem. 1978;40(1):15–21.
Gross KA, Berndt CC. Thermal processing of hydroxyapatite for coating production. J Biomed Mater Res. 1998;39(4):580–7.
Trębacz H, Wójtowicz K. Thermal stabilization of collagen molecules in bone tissue. Int J Biol Macromol. 2005;37(5):257–62.
Matsunaga K, Murata H. Formation energies of substitutional sodium and potassium in hydroxyapatite. Mater Tran. 2009;50(5):1041–5.
Etok SE, Valsami-Jones E, Wess TJ, Hiller JC, Maxwell CA, Rogers KD, Manning DAC, White ML, Lopez-Capel E, Collins MJ, Buckley M, Penkman KEH, Woodgate SL. Structural and chemical changes of thermally treated bone apatite. J Mater Sci. 2007;42(23):9807–16.
Acknowledgements
S. M. Londoño-Restrepo thanks the Consejo Nacional de Ciencia y Tecnologıa (CONACYT-Mexico) for the financial support of her Ph.D. thesis, and PAPIIT project number IN112317 for the financial support of this investigation as well. The authors would like to thank Dr. Genoveva Hernandez-Padrón for her technical support for the Raman experiments, Dra. Beatriz Millan Malo and M. Alicia del Real for technical support, and Carolina Muñoz from CGEO-UNAM for the ICP determinations. Authors thank Alexandra Morin Dube for the technical English revision of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Londoño-Restrepo, S.M., Jeronimo-Cruz, R., Rubio-Rosas, E. et al. The effect of cyclic heat treatment on the physicochemical properties of bio hydroxyapatite from bovine bone. J Mater Sci: Mater Med 29, 52 (2018). https://doi.org/10.1007/s10856-018-6061-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6061-5