Abstract
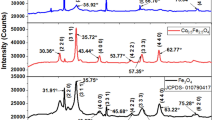
The manufacture of nanotechnology is evolving quickly, and it is anticipated that the key developments will have a substantial impact on both business and science and be applicable in a wide range of fields. Cobalt ferrite (CoFe2O4) nanoparticles have been thought of as one of the top contenders in this field. The goal of the current work is to determine how the chelating agent affects on the physical properties of Nd+ 3-doped CoFe2O4 (CFO) nanomaterial. Sol–gel auto-combustion method with different chelating agents such as oxalic acid (OA), citric acid (CA), and ethylenediamine tetraacetic acid (EDTA) was used to prepare the Nd+ 3 ion-doped cobalt ferrites with the formula CoNd0.075Fe1.925O4(CNFO). X-ray diffraction (XRD) data gathered from the synthesized samples reveal that they have a single-phase spinel structure without any impurity phase or unreacted starting materials. The average particle sizes 28, 35, and 19 nm were observed through scanning electron microscopy (SEM) for the CNFO samples obtained from OA, CA, and EDTA, respectively. The magnetization value of CNFO obtained from EDTA is 76.79 emu/g, which is superior to that of CNFO obtained from OA (58.48 emu/g) and CA (61.12 emu/g). Temperature-dependent (in the range of 300-500 K) dielectric constant, dielectric loss, and electrical conductivity are also investigated as a function of frequency. The calculated grain boundary activation energy for the CNFO nanomaterial obtained from EDTA is 0.64 eV, and which is higher than that of OA (0.54 eV) and CA (0.58 eV). The nano-sized CNFO synthesized using the EDTA as a chelating agent having the lesser crystallite size (19 nm) and high magnetization (76.79 emu/g) as well as high coercivity (2917 Oe), and hence, it can be used for magnetic data storage and magneto-recording systems.









Similar content being viewed by others

Data availability
The authors declare that all the data generated or analyzed during this study are included in this manuscript
References
C.P. Luo, S.H. Liou, L. Gao, Y. Liu, D.J. Sellmyer, Nanostructured FePt: B2O3 thin films with perpendicular magnetic anisotropy. Appl. Phys. Lett. (2000). https://doi.org/10.1063/1.1314289
C.V. Gopal Reddy, S.V. Manorama, V.J. Rao, Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sens. Actuators B Chem. 55, 90–95 (1999). https://doi.org/10.1016/s0925-4005(99)00112-4
A. Abbasi, D. Ghanbari, M. Salavati-Niasari, M. Hamadanian, Photo-degradation of methylene blue: photocatalyst and magnetic investigation of Fe2O3–TiO2 nanoparticles and nanocomposites. J. Mater. Sci. Mater. Electron. 27, 4800–4809 (2016). https://doi.org/10.1007/s10854-016-4361-4
M.J. Uddin, Y.-K. Jeong, Application of magnesium ferrite nanomaterials for adsorptive removal of arsenic from water: effects of mg and Fe ratio. Chemosphere 307, 135817 (2022). https://doi.org/10.1016/j.chemosphere.2022.135817
L. Zhao, X. Li, Q. Zhao, Z. Qu, D. Yuan, S. Liu, X. Hu, G. Chen, Synthesis, characterization and adsorptive performance of MgFe2O4nanospheres for SO2 removal. J. Hazard. Mater. 184, 704–709 (2010). https://doi.org/10.1016/j.jhazmat.2010.08.096
O. Mounkachi, R. Lamouri, B. Abraime, H. Ez-Zahraouy, A. ElKenz, M. Hamedoun, A. Benyoussef, Exploring the magnetic and structural properties of Nd-doped Cobalt nano-ferrite for permanent magnet applications. Ceram. Int. 43, 14401–14404 (2017). https://doi.org/10.1016/j.ceramint.2017.07.209K.-X
F. Shi, K.X. Shi, F. Qiu, P. Wang, H. Li, C.C. Wang, Magnetic MgFe2O4/MIL-88A catalyst for photo-Fenton sulfamethoxazole decomposition under visible light. Sep. Purif. Technol. (2022). https://doi.org/10.1016/j.seppur.2022.121965
H. Wu, G. Wu, L. Wang, Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: facile synthesis and electromagnetic properties. Powder Technol. 269, 443–451 (2015). https://doi.org/10.1016/j.powtec.2014.09.045
G. Wu, H. Zhang, X. Luo, L. Yang, H. Lv, Investigation and optimization of Fe/ZnFe2O4 as a wide-band electromagnetic absorber. J. Colloid Interf. Sci. 536, 548–555 (2019). https://doi.org/10.1016/j.jcis.2018.10.084
H. Zhang, Z. Jia, A. Feng, Z. Zhou, L. Chen, C. Zhang, X. Liu, G. Wu, In situ deposition of pitaya-like Fe3O4@C magnetic microspheres on reduced graphene oxide nanosheets for electromagnetic wave absorber. Compos. Part B Eng. 199, 108261 (2020). https://doi.org/10.1016/j.compositesb.2020.108261
X. Zhou, B. Wang, Z. Jia, X. Zhang, X. Liu, K. Wang, B. Xu, G. Wu, Dielectric behavior of Fe3N@C composites with green synthesis and their remarkable electromagnetic wave absorption performance. J. Colloid Interf. Sci. 582, 515–525 (2021). https://doi.org/10.1016/j.jcis.2020.08.087
M.A. Almessiere, Y. Slimani, A.V. Trukhanov, A. Baykal, H. Gungunes, E.L. Trukhanov, S.V. Trukhanov, V.G. Kostishin, Strong correlation between Dy3+ concentration, structure, magnetic and microwave properties of the [Ni0.5Co0.5](DyxFe2-x)O4 nanosized ferrites. J. Ind. Eng. Chem. 90, 251–259 (2020). https://doi.org/10.1016/j.jiec.2020.07.020
D.P. Sherstyuk, A. Yu Starikov, V.E. Zhivulin, D.A. Zherebtsov, S.A. Gudkova, N.S. Perov, Y.A. Alekhina, K.A. Astapovich, D.A. Vinnik, A.V. Trukhanov, Effect of Co content on magnetic features and SPIN states IN Ni–Zn spinel ferrites. Ceram. Int. 47, 12163–12169 (2021). https://doi.org/10.1016/j.ceramint.2021.01.063
D.A. Vinnik, V.E. Zhivulin, D.P. Sherstyuk, A.Y. Starikov, P.A. Zezyulina, S.A. Gudkova, D.A. Zherebtsov, K.N. Rozanov, S.V. Trukhanov, K.A. Astapovich, A.S.B. Sombra, D. Zhou, R.B. Jotania, C. Singh, A.V. Trukhanov, Ni substitution effect on the structure, magnetization, resistivity and permeability of zinc ferrites. J. Mater. Chem. C (2021). https://doi.org/10.1039/d0tc05692h
M.A. Almessiere, Y. Slimani, H. Güngüne, S. Ali, A. Manikandan, I. Ercan, A. Baykal, A.V. Trukhanov, Magnetic attributes of NiFe2O4 nanoparticles: influence of dysprosium Ions (Dy3+) substitution. Nanomaterials 9, 820 (2019). https://doi.org/10.3390/nano9060820
V.A. Ketsko, E.N. Beresnev, M.A. Kopeva, L.V. Elesina, A.I. Baranchikov, A.I. Stognii, A.V. Trukhanov, N.T. Kuznetsov, Specifics of pyrohydrolytic and solidphase syntheses of solid solutions in the (MgGa2O4)x(MgFe2O4)1 – x system. Russ. J. Inorg. Chem. 55, 427–429 (2010). https://doi.org/10.1134/S0036023610030216
Y. Slimani, B. Unal, M.A. Almessiere, A. Demir Korkmaz, S.E. Shirsath, G. Yasin, A.V. Trukhanov, A. Baykal, Investigation of structural and physical properties of Eu3+ ions substituted Ni0.4Cu0.2Zn0.4Fe2O4 spinel ferrite nanoparticles prepared via sonochemical approach. Results Phys. 17, 103061 (2020). https://doi.org/10.1016/j.rinp.2020.103061
M.A. Almessiere, Y. Slimani, H. Güngüneş, V.G. Kostishyn, S.V. Trukhanov, A.V. Trukhanovd, A. Baykal, Impact of Eu3+ ion substitution on structural, magnetic and microwave traits of Ni–Cu–Zn spinel ferrites. Ceram. Int. 46, 11124–11131 (2020). https://doi.org/10.1016/j.ceramint.2020.01.132
S.R. Sivagurunathan, Gibin, Preparation and characterization of nanosized cobalt ferrite particles by co-precipitation method with citrate as chelating agent. J. Mater. Sci: Mater. Electron. 27, 8891–8898 (2016). https://doi.org/10.1007/s10854-016-4915-5
T.L. Templeton, A.S. Arrott, A.E. Curzon, M.A. Gee, X.-Z. Li, Y. Yoshida, P.J. Schurer, J.L. LaCombe, Magnetic properties of CoxFe3 – xO4 during conversion from normal to inverse spinel particles. J. Appl. Phys. 73, 6728 (1993). https://doi.org/10.1063/1.352516P
S. Ayyappan, S. Mahadevan, P. Chandramohan, M.P. Srinivasan, J. Philip, B. Raj, Influence of Co2+ Ion Concentration on the size, magnetic properties, and purity of CoFe2O4 spinel ferrite nanoparticles. J. Phys. Chem. C 114, 6334–6341 (2010). https://doi.org/10.1021/jp911966p
R. Zhang, L. Suna, Z. Wang, W. Hao, E. Cao, Y. Zhang, Dielectric and magnetic properties of CoFe2O4 prepared by sol-gel auto-combustion method. Mater. Res. Bull. 98, 133–138 (2018). https://doi.org/10.1016/j.materresbull.2017.08.006
C. Murugesan, L. Okras, K. Ugendar, G. Chandrasekaran, X. Liu, D. Diao, J. Shen, Improved magnetic and electrical properties of zn substituted nanocrystalline MgFe2O4 ferrite. J. Magn. Magn. Mater. 550, 169066 (2022). https://doi.org/10.1016/j.jmmm.2022.169066
G. Bulai, L. Diamandescu, I. Dumitru, S. Gurlui, M. Feder, O.F. Caltun, Effect of rare earth substitution in cobalt ferrite bulk materials. J. Magn. Magn. Mater. (2015). https://doi.org/10.1016/j.jmmm.2015.04.089
M. Krishna Surendra, D. Kannan, M.S. Ramachandra Rao, Magnetic and dielectric properties study of cobalt ferrite nanoparticles synthesized by co-precipitation method. MRS Proc. 1368, 1140 (2011). https://doi.org/10.1557/opl.2011.1281
G. Allaedini, S.M. Tasirin, P. Aminayi, Magnetic properties of cobalt ferrite synthesized by hydrothermal method. Int. Nano Lett. 5, 183–186 (2015). https://doi.org/10.1007/s40089-015-0153-8
I. Sharifi, H. Shokrollahi, M.M. Doroodmand, R. Safi, Magnetic and structural studies on CoFe2O4 nanoparticles synthesized by co-precipitation, normal micelles and reverse micelles methods. J. Magn. Magn. Mater. 324, 1854–1861 (2012). https://doi.org/10.1016/j.jmmm.2012.01.015
Y.I. Kim, D. Kim, C.S. Lee, Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled co-precipitation method. Phys. B: Condens. Matter. 337, 42–51 (2003). https://doi.org/10.1016/S0921-4526(03)00322-3
Z.T. Chen, L. Gao, Synthesis and magnetic properties of CoFe2O4 nanoparticles by using PEG as surfactant additive. Mater. Sci. Eng. B 141, 82 (2007). https://doi.org/10.1016/j.mseb.2007.06.003
X. Jia, D. Chen, X. Jiao, T. He, H. Wang, W. Jiang, Monodispersed Co, Ni-Ferrite nanoparticles with tunable sizes: controlled synthesis, magnetic properties, and surface modification. J. Phys. Chem. C 112, 911 (2008). https://doi.org/10.1021/jp077019+
L. Kumar, M. Kar, Effect of substitution on the structural and magneto crystalline anisotropy of nanocrystalline cobalt ferrite (CoFe2−xLaxO4) . Ceram. Int. 38, 4771–4782 (2012). https://doi.org/10.1016/j.ceramint.2012.02.065
Y. Slimani, M.A. Almessiere, A. Demir Korkmaz, A. Baykal, H. Gungunes, M.G. Vakhitov, D.S. Klygach, S.V. Trukhanovi, A.V. Trukhanov, The impact of indium ion on structural, magnetic, and electrodynamic traits of co-ni nanospinel ferrites. J. Magn. Magn. Mater. 562, 169782 (2022). https://doi.org/10.1016/j.jmmm.2022.169782
B. Ünal, M.A. Almessiere, A. Demir Korkmaz, Y. Slimani, A. Baykal, Effect of thulium substitution on conductivity and dielectric belongings of nanospinel cobalt ferrite. J. Rare Earths 38, 1103–1113 (2020). https://doi.org/10.1016/j.jre.2019.09.011
M.A. Almessiere, Y. Slimani, A.D. Korkmaz, S. Guner, M. Sertkol, S.E. Shirsath, A. Baykal, Structural, optical and magnetic properties of Tm3+ substituted cobalt spinel ferrites synthesized via sonochemical approach. Ultrason. Sonochem. 54, 1–10 (2019). https://doi.org/10.1016/j.ultsonch.2019.02.022
S. Akhtar, Y. Slimani, M.A. Almessiere, A. Baykal, E. Gokce Polat, S. Caliskan, Influence of Tm and Tb co-substitution on structural and magnetic features of CoFe2O4 nanospinel ferrites. Nano-Struct. Nano-Obj. 33, 100944 (2023). https://doi.org/10.1016/j.nanoso.2023.100944
Y. Slimani, M.A. Almessiere, S. Guner, B. Aktas, S.E. Shirsath, M.V. Silibin, A.V. Trukhanov, A. Baykal, Impact of Sm3+ and Er3+ cations on the structural, optical, and magnetic traits of spinel cobalt ferrite nanoparticles: comparison investigation. ACS Omega (2022). https://doi.org/10.1021/acsomega.1c06898
A.K. Nikumbh et al., Structural, electrical, magnetic and dielectric properties of rare-earth substituted cobalt ferrites nanoparticles synthesized by the co-precipitation method. J. Magn. Magn. Mater. 355, 201–209 (2014). https://doi.org/10.1016/j.jmmm.2013.11.052
E.E. Ateia, M.K. Abdelmaksoud, M.M. Arman, A.S. Shafaay, Comparative study on the physical properties of rare–earth–substituted nano–sized CoFe2O4. Appl. Phys. A 126, 91 (2020). https://doi.org/10.1007/s00339-020-3282-5
F.X. Cheng, J.T. Jia, Z.G. Xu, B. Zhou, C.S. Liao, C.H. Yan, L.Y. Chen, H.B. Zhao, Microstructure, magnetic, and magneto-optical properties of chemical synthesized Co-RE (RE = Ho, Er, Tm, Yb, Lu) ferrite nanocrystalline films. J. Appl. Phys. 86, 2727–2732 (1999). https://doi.org/10.1063/1.371117
R.S. Yadav, J. Havlica, J. Masilko, L. Kalina, J. Wasserbauer, M. Hajdúchová, V. Enev, I. Kuřitka, Z. Kožákov, Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J. Magn. Magn. Mater. 399, 109–117 (2016). https://doi.org/10.1016/j.jmmm.2015.09.055
L.B. Tahar, M. Artus, S. Ammar, L.S. Smiri, F. Herbst, M.-J. Vaulay, V. Richard, J.-M. Grenèche, F. Villain, F. Fiévet, Magnetic properties of CoFe19RE0.1O4 nanoparticles (RE = La, Ce, Nd, Sm, Eu, Gd, Tb, Ho) prepared in polyol. J. Magn. Magn. Mater. 320, 3242–3250 (2008). https://doi.org/10.1016/j.jmmm.2008.06.031
Y.H. Hou, Y.L. Huang, S.J. Hou, S.C. Ma, Z.W. Liu, Y.F. Ouyang, Structural, electronic and magnetic properties ofRE3+-doping in CoFe2O4: a first-principles study. J. Magn. Magn. Mater. 421, 300–305 (2017). https://doi.org/10.1016/j.jmmm.2016.08.027
L. Zhao, H. Yang, X.P. Zhao, L.X. Yu, Y.M. Cui, S.H. Feng, Magnetic properties of CoFe2O4 ferrite doped with rare earth ion. Mater. Lett. 60, 1–6 (2006). https://doi.org/10.1016/j.matlet.2005.07.017
K. Mohit, S.K. Rout, S. Parida, G.P. Singh, S.K. Sharma, S.K. Pradhan, I.W. Kim, Structural, optical and dielectric studies of NixZn1–xFe2O4 prepared by auto combustion route. Phys. B: Condens. Matter. 407, 935–942 (2012). https://doi.org/10.1016/j.physb.2011.12.003
P. Gupta, R. Bhargava, R. Das, P. Poddar, Static and dynamic magnetic properties and effect of surface chemistry on the morphology and crystallinity of DyCrO3 nanoplatelets. RSC Adv. 3, 26427–26432 (2013). https://doi.org/10.1039/C3RA43088J
R. Rai, M. Molli, Effect of different complexing agents on the magnetic, optical and photocatalytic properties of sol–gel synthesized KBiFe2O5. Bull. Mater. Sci. 44, 34 (2021). https://doi.org/10.1007/s12034-020-02328-8
C. Venkatrao, D.R.S. Reddy, K.R. Kandula, R. Bhimireddi, G.S.-D. Structural, Optical, dielectric, and magnetic properties of YFeO3 Nanomaterials obtained by the sol-gel technique using tartaric acid as a chelating agent. Phys. Status Solidi.(b) (2022). https://doi.org/10.1002/pssb.202200272
W.C. Kim, S.W. Lee, S.J. Kim, S.H. Yoon, C.S. Kim, Magnetic properties of Y-, La-, Nd-, Gd-, and bi-doped ultrafine CoFe2O4 spinel grown by using a sol-gel method. J. Magn. Magn. Mater. (2000). https://doi.org/10.1016/S0304-8853(00)00121-9
S. Amiri, H. Shokrollahi, Magnetic and structural properties of RE doped co-ferrite (RE = nd, Eu, Gd) nano-particles synthesized by co-precipitation. J. Mag. Magn. Mater 345, 18–23 (2013). https://doi.org/10.1016/j.jmmm.2013.05.030
M. Ahmadipour, M.J. Abu, M.F. Ab Rahman, M.F. Ain, Z.A. Ahmad, Assessment of crystallite size and strain of CaCu3Ti4O12 prepared via conventional solid‐state reaction. Micro Nano Lett. 11, 147–150 (2016). https://doi.org/10.1049/mnl.2015.0562
S. Jabez, S. Mahalakshmi, S. Nithiyanantham, Frequency and temperature effects on dielectric properties of cobalt ferrite. J. Mater. Sci: Mater. Electron. 28, 5504–5511 (2017). https://doi.org/10.1007/s10854-016-6212-8
A. Ghasemi, M. Mousavinia, Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol–gel method. Ceram. Int. 40, 2825–2834 (2014). https://doi.org/10.1016/j.ceramint.2013.10.031
A. Goktas, Role of simultaneous substitution of Cu2+ and Mn2+ in ZnS thin films: defects-induced enhanced room temperature ferromagnetism and photoluminescence. Physica E 117, 113828 (2020). https://doi.org/10.1016/j.physe.2019.113828
F. Mikailzade, H. Türkan, F. Önal, M. Zarbali, A. Göktaş, A. Tumbul, Structural and magnetic properties of polycrystalline Zn1 – xMnxO films synthesized on glass and p–type Si substrates using Sol–Gel technique. Appl. Phys. A 127, 408 (2021). https://doi.org/10.1007/s00339-021-04519-4
R. Jabbar, S.H. Sabeeh, A.M. Hameed, Structural, dielectric and magnetic properties of Mn+ 2 doped cobalt ferrite nanoparticles. J. Magn. Magn. Mater. 494, 165726 (2020). https://doi.org/10.1016/j.jmmm.2019.165726
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
NBG: Sample preparation and their structural characterization and original draft preparation. RSRD: Conceptualization, and Reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gatchakayala, N.B., Dachuru, R.S.R. Influence of chelating agent on structural, magnetic, and dielectric properties of CoNd0.075Fe1.925O4-nanosized spinels ferrites derived from sol–gel auto-combustion method. J Mater Sci: Mater Electron 34, 1394 (2023). https://doi.org/10.1007/s10854-023-10794-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10794-z