Abstract
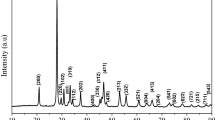
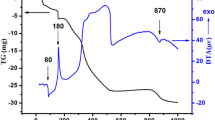
The perovskite SrTiO3−δ elaborated by chemical route was characterised by physical and electrochemical methods. The oxide crystallizes in a cubic structure with a lattice parameter of 3.907(5) Å and a crystallite dimension of 71 nm. The morphology visualized by the SEM-EDX analysis shows spherical grains with a mean size of 0.2 μm, formed by agglomeration of crystallites. The diffuse reflectance gave a gap (Eg) of 3.21 eV, ascribed to the charge transfer O2−: 2p → Ti4+: 3d. The thermal variation of the conductivity (σ) obeys an exponential law {σ = σoexp-0.27 eV/T} assigned to low hopping polarons. The electrochemical characterization was undertaken in Na2SO4 solution (10− 1 M) and the n-type character demonstrated from the capacitance measurement and cyclic voltammetry under UV-irradiation, is due to oxygen deficiency. A flat band potential (Efb) of − 0.31 VSCE was determined with a depletion width extended up to ~ 4 μm, advantageous for the formation of electron / hole (e− / h+) pairs. The electrochemical stability against photocorrosion was also shown from the semi-logarithmic plot (log J–E). As application, the Congo Red a diazo dye (CR) is successfully oxidized under UV light on SrTiO3−δ; an abatement of 63% was reached. According to the scavengers mechanism, hydroxyl •OH is the main specie responsible for the CR oxidation. The discoloration follows a pseudo-first-order kinetic with a half-life of 189 min.












Similar content being viewed by others
Data availability
All data generated in this work are analyzed in this work are included in the submitted article.
References
N. Boutal, G. Rekhila, K. Taibi, M. Trari, Relaxor ferroelectric and photo- electrochemical properties of lead-free Ba1xEu2x/3(Ti0.75Zr0.25)O3 ceramics, application to chromate reduction. J. Sol. Energy 99, 291–298 (2014). https://doi.org/10.1016/j.solener.2013.11.019
M. Ismail, N.A. Sazelee, N.A. Ali, S. Suwarno, Catalytic effect of SrTiO3 on the dehydrogenation properties of LiAlH4. J. Alloys Compd. 855, 157475 (2021). https://doi.org/10.1016/j.jallcom.2020.157475
B. Gulen, P. Demircivi, E.B. Simsek, UV-A light irradiated photocatalytic performance of hydrothermally obtained W doped BaZrO3 catalyst against the degradation of levofloxacin and tetracycline antibiotic. J. Photochem. Photobiol. A 404, 112869 (2021). https://doi.org/10.1016/j.jphotochem.2020.112869
F. Moulai, O. Fellahi, B. Messaoudi, O. Fellahi, T. Hadjersi, L. Zerroual, Electrodeposition of nanostructured γ-MnO2 film for photodegradation of rhodamine B. Ionics 24, 2099–2109 (2018). https://doi.org/10.1007/s11581-018-2440-7
L. Boudjellal, A. Belhadi, R. Brahimi, S. Boumaza, M. Trari, Semiconducting and photoelectrochemical properties of the ilmenite CoTiO3 prepared by wet method and its application for O2 evolution under visible light. J. Solid State Electrochem. 24, 357–364 (2020). https://doi.org/10.1007/s10008-019-04464-6
R.N. Perumal, V. Athikesavan, Structural and electrical properties of lanthanidedoped Bi0.5(Na0.80K0.20)0.5TiO3–SrZrO3 piezoelectric ceramics for energystorage applications. J. Mater. Sci. Mater. Electron. 31, 4092–4105 (2020). https://doi.org/10.1007/s10854-020-02956-0
N. Bensemma, N. Boutal, K. Taibi, M. Trari, Some lead-free ceramics of ferroelectric and photoelectrochemical applications. ISPDS1 (2016). https://doi.org/10.23647/ca.md20171501
K. Yu, C. Zhang, Y. Chang, Y. Feng, Z. Yang, L. Lou, S. Luib, Novel threedimensionally ordered macroporous SrTiO3 photocatalysts with remarkably enhanced hydrogen production performance. Appl. Catal. B: Environ. 200, 514–520 (2017). https://doi.org/10.1016/j.apcatb.2016.07.049
S. Patial, V. Hasija, P. Raizada, P. Singh, A.A.P. Khan Singh, A.M. Asiri, Tunable photocatalytic activity of SrTiO3 for water splitting: strategies and future scenario. J. Environ. Chem. Eng. 8, 103791 (2020). https://doi.org/10.1016/j.jece.2020.103791
N.Y. Devi, P. Rajasekaran, K. Vijayakumar, A.S.A. Nedunchezhian, D. Sidharth, G. Anbalagan, M. Arivanandhan, R. Jayavel, Enhancement of thermoelectric power factor of hydrothermally synthesised SrTiO3 nanostructures. Mater. Res. Express. 7, 015094 (2020). https://doi.org/10.1088/2053-1591/ab6c96
H.A.S.A. Yurtseven, A. N, Kiraci, Damping constant and the inverse relaxation time calculated as a function of pressure using the X-ray diffraction data close to the cubic-tetragonal phase transition in SrTiO3. Ferroelectrics 551, 143–151 (2019). https://doi.org/10.1080/00150193.2019.1658041
K.R. Thampi, M.S. Rao, W. Schwarz, M. Gratzel, J. Kiwi, Preparation of SrTiO3, for the Photoinduced by Sol-Gel Techniques Production of H2, and Surface Peroxides from Water. J. Chem. Sac Faraday Trans. I 84, 1703–1712 (1988). https://doi.org/10.1039/F19888401703
S. Boumaza, A. Boudjemaa, A. Bouguelia, R. Bouarab, M. Trari, Visible light induced hydrogen evolution on new hetero-system ZnFe2O4/SrTiO3. Appl. Energy 87, 2230–2236 (2010). https://doi.org/10.1016/j.apenergy.2009.12.016
R. Bagtache, I. Sebai, M. Trari, Visible light induced H2 evolution on the hetero-junction Pt/CuCo2O4 prepared by hydrothermal route. J. Sol. Energy 211, 971–976 (2020). https://doi.org/10.1016/j.solener.2020.10.046
K. Iwashina, A. Kudo, Rh-Doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-light irradiation. J. Am. Chem. Soc 133, 13272–13275 (2011). https://doi.org/10.1021/ja2050315
R. Konta, H. IshiiT, Kato, A. Kudo, Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation. J. Phys. Chem. B 108, 8992–8995 (2004). https://doi.org/10.1021/jp049556p
S. Omeiri, B. Hadjarab, A. Bouguelia, M. Trari, Electrical, optical and photoelectrochemical properties of BaSnO3 – I applications to hydrogen evolution. J. Alloys Compd. 505, 592–597 (2010). https://doi.org/10.1016/j.jallcom.2010.06.081
S. Wang, H. Gao, X. Yu, S. Tang, Y. Wang, L. Fang, X. Zhao, J. Li, L. Yang, W. Dang, Nanostructured SrTiO3 with different morphologies achieved by mineral acid-assisted hydrothermal method with enhanced optical, electrochemical, and photocatalytic performances. J. Mater. Sci. Mater. 31, 17736–17754 (2020). https://doi.org/10.1007/s10854-020-04328-0
H. Gao, H. Yang, S. Wang, Hydrothermal synthesis, growth mechanism, optical properties and photocatalytic activity of cubic SrTiO3 particles for the degradation of cationic and anionic dyes. Optik 175, 237–249 (2018). https://doi.org/10.1016/j.ijleo.2018.09.027
G. Campet, M. Carrere, C. Puprichitkun, S.Z. Wen, J. Salardenne, J. Claverie, N-Type SrTiO3 thin films: electronic processes and photoelectrochemical behaviour. J. Solid State Chem. 69, 267–273 (1987). https://doi.org/10.1016/0022-4596(87)90083-1
H. Gerischer, Charge transfer processes at semiconductor-electrolyte interfaces in connection with problems of catalysis. Surf. Sci. 18, 97–122 (1969). https://doi.org/10.1016/0039-6028(69)90269-6
H. Geriscber, A. Heller, The role of oxygen in photooxidationof organic molecules on semiconductor particles. J. Phys. Chem. 95, 5261–5267 (1991). https://doi.org/10.1021/j100166a063
H. Geriscber, Heller, A photocatalytic oxidation of organic molecules at TiO2 particles by sunlight in aerated water. J. Electrochem. Soc. 139, 113 (1992). https://doi.org/10.1149/1.2069154
F. Saib, B. Bellal, M. Trari, Preparation and characterization of the brownmillerite Sr2Co2O5 as novel photocatalyst in the hydrogen generation. Mater. Sci. Semicond. Process. 63, 122–126 (2017). https://doi.org/10.1016/j.mssp.2016.12.044
V. Berbenni, A. Marini, G. Bruni, Effect of mechanical activation on the preparation of SrTiO3 and Sr2TiO4 ceramics from the solid state system SrCO3–TiO2. J. Alloys Compd. 329, 230–238 (2001). https://doi.org/10.1016/S0925-8388(01)01574-2
P. Jayabal, V. Sasirekha, J. Mayandi, K. Jeganathan, V. Ramakrishnan, A facile hydrothermal synthesis of SrTiO3 for dye sensitized solar cell application. J. Alloys Compd. 586, 456–461 (2014). https://doi.org/10.1016/j.jallcom.2013.10.012
J.I. Fujisawa, T. Eda, M. Hanaya, Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 685, 23–26 (2017). https://doi.org/10.1016/j.cplett.2017.07.031
J.Y. Baek, L.T. Duy, S.Y. Lee, H. Seo, Aluminum doping for optimization of ultrathin and high-k dielectric layer based on SrTiO3. Mater. Sci. Technol. 42, 28–37 (2020). doi:https://doi.org/10.1016/j.jmst.2019.12.006
M.L. Moreira, V.M. Longo, A. Jr., W., Ferrer, M.M. Andres, J. Mastelaro, V.R. Varela, J.A. Longo E, Quantum mechanics insight into the microwave nucleation of SrTiO3 nanospheres. J. Phys. Chem. C 116, 24792–24808 (2012). https://doi.org/10.1021/jp306638r. |
S.E. Reyes-Lillo, T. Rangel, F. Bruneval, J.B. Neaton, Effects of quantum confinement on excited state properties of SrTiO3 from ab initio many-body perturbation theory. Phys. Rev. B 94, 041107 (2016). https://doi.org/10.1103/PhysRevB.94.041107
B. Hadjarab, M. Trari, M. Kebir, Physical characterization of the semiconducting deficient perovskite BaSnO3–δ. Mater. Sci. Semicond. Process. 29, 283–287 (2015). https://doi.org/10.1016/j.mssp.2014.04.041i
S. Boumaza, L. Boudjellal, R. Brahimi, A. Belhadi, M. Trari, Synthesis by citrates sol-gel method and characterization of the perovskite LaFeO3: application to oxygen photo-production. J. Sol-Gel Sci. Technol. 94, 486–492 (2020). https://doi.org/10.1007/s10971-020-05275-2
A. Sahmi, R. Laib, S. Omeiri, K. Bensadok, M. Trari, Photoelectrochemical properties of the perovskite BaSnO3 synthetized by chemical route. Application to electro-photocatalytic mineralization of ibuprofen. J. Photochem Photobiol. A 364, 443–448 (2018). https://doi.org/10.1016/j.jphotochem.2018.06.034
H. Fu, Electron transport in SrTiO3 accumulation layers and semiconductor nanocrystal films. Ph. D Thesis (University of Minnesota), pp.19, (2017)
A. Boudjemaaa, S. Boumazaa, M. Trari, R. Bouarab, Bouguelia, A physical and photo-electrochemical characterizations of a-Fe2O3, application for hydrogen production. Int. J. Hydrog Energy 344, 268–4274 (2009). doi:https://doi.org/10.1016/j.ijhydene.2009.03.044
M. Trari, J.P. Doumerc, P. Dordor, M. Pouchard, G. Behr, G. Krabbes, Preparation and characterization of lanthanum doped BaSnO3. J. Phys. Chem. Solids 55, 1239–1243 (1994). https://doi.org/10.1016/0022-3697(94)90205-4
R.R. Nasby, R.K. Quinn, Photoacocted electrolytes of water using a BaTiO3 electrode. Mater. Res. Bull. 11, 985–992 (1976). https://doi.org/10.1016/0025-5408(76)90174-4
S. Boumaza, R. Brahimi, L. Boudjellal, A. Belhadi, M. Trari, Photoelectrochemical study of La2NiO4 synthesized using citrate sol gel method—application for hydrogen photo-production. J. Solid State Electrochem. 24, 329–337 (2020). https://doi.org/10.1007/s10008-019-04470-8
H. Bouakaz, M. Abbas, R. Brahimi, M. Trari, Physical properties of the delafossite CuCoO2 synthesized by co-precipitation/hydrothermal route. Mater. Sci. Semicond. Process. 136, 106132 (2021). https://doi.org/10.1016/jmssp2021106132
H. Lahmar, M. Benamira, S. Douafer, F. Akika, M. Z, Hamdi, I. Avramova, M. Trari, Photocatalytic degradation of Crystal violet dye on the novel CuCr2O4/SnO2 hetero-system under sunlight. Optik 219, 165042 (2020). https://doi.org/10.1016/jijleo2020165042
M. Abbas, M. Trari, Contribution of adsorption and photo catalysis for the elimination of black eriochrome (NET) in an aqueous medium-optimization of the parameters and kinetics modeling. Sci. Afr. 8, e00387 (2020). https://doi.org/10.1016/j.sciaf.2020.e00387
W.A. Aboutaleb, R.A. El-Salamony, Effect of Fe2O3-CeO2 nanocomposite synthesis method on the Congo red dye photodegradation under visible light irradiation. Mater. Chem. Phys. 236, 121724 (2019). https://doi.org/10.1016/j.matchemphys.2019.121724
B. Boutra, N. Güy, M. Özacar, M. Trari, Magnetically separable MnFe2O4/TA/ZnO nanocomposites for photocatalytic degradation of Congo red under visible light. Magn. Magn. Mater. 497, 165994 (2020). https://doi.org/10.1016/j.jmmm.2019.165994
Y.H. Chiu, T.F.M. Chang, C.Y. Chen, M. Sone, Y.J. Hsu, Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 9, 430 (2019). https://doi.org/10.3390/catal9050430
Acknowledgements
The authors are grateful to Dr K. khenfer for the XRD analysis.
Funding
The financial support of this work was provided by the Universities of Boumerdes (UMBB) and Algiers (USTHB), Algeria).
Author information
Authors and Affiliations
Contributions
SM: performed the experiments, wrote and discussed the results, MA: Visualization and Editing the work, RB: performed electrochemical experiments and Formal analysis, BB: performed and discussed the optical results, MT: Validation of the manuscript and supervised the results.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Merrad, S., Abbas, M., Brahimi, R. et al. Physical properties of the perovskite SrTiO3−δ synthetized by chemical route. J Mater Sci: Mater Electron 34, 206 (2023). https://doi.org/10.1007/s10854-022-09616-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09616-5