Abstract
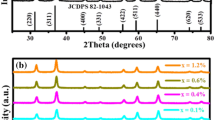
The pure and Eu2+-doped NaCl crystal were successfully grown by the melt (Czochralski) method. The structural, mechanical and optical properties were characterized by carrying out X-ray diffraction (XRD), micro-hardness, optical absorption, photoluminescence (PL) and thermoluminescence (TL) measurements. The XRD analysis indicates that the Eu2+ ions have been incorporated into the NaCl crystal matrix and the diffraction peak shifts toward lower 2θ angle. Hardness measurement has shown a decrease of crystal hardness with indentation load. Optical absorption spectrum has shown the presence of peaks at 211, 224, 231, 255, 320 and 352 nm. Results of PL analysis indicate that the emission peak exists the characteristic peak of bivalent Eu ions corresponding to the t2g(4f65d) → 8S7/2(4f7) transition. The TL glow curve of NaCl:Eu2+ crystal has shown two peaks located at 108.6 and 199.6 °C with different trap depth, the intensity of the high-temperature peak is found to be larger than the low-temperature one.





Similar content being viewed by others
References
D.B. Sirdeshmukh, L. Sirdeshmukh, K.G. Subhadra, Alkali Halides (Springer, Berlin, 2001)
H.W. Joo, H.S. Park, J.H. Kim, J.Y. Lee, S.K. Kim, Y.D. Kim, H.S. Lee, S.H. Kim, Quenching factor measurement for NaI(Tl) scintillation crystal. Astropart. Phys. 108, 50–56 (2019). https://doi.org/10.1016/j.astropartphys.2019.01.001
Y. Li, Y. Li, Z. Yang, X. Zhang, F. Zeng, C. Li, H. Lin, Z. Su, C.K. Mahadevan, J. Liu, The structure and liquid flow effect of melt during NaCl crystal growth. Cryst. Res. Technol. (2020). https://doi.org/10.1002/crat.201900229
Y. Li, Y. Li, Z. Yang, X. Zhang, J. Liu, F. Zeng, J. Yao, C. Li, H. Lin, Z. Su, C.K. Mahadevan, Structural, optical and mechanical properties and cracking factors of large-sized KBr:Ce3+ single crystal. J. Electron. Mater. 49, 4785–4793 (2020). https://doi.org/10.1007/s11664-020-08173-z
Y. Li, Y. Li, C. Li, X. Zhang, F. Zeng, H. Lin, Z. Su, C.K. Mahadevan, Luminescent and mechanical properties of cerium doped potassium chloride single crystal. Cryst. Res. Technol. (2020). https://doi.org/10.1002/crat.202000060
Y. Li, Y. Li, F. Meng, X. Zhang, F. Zeng, J. Liu, H. Liu, J. Wang, C.K. Mahadevan, Effect of cooling time on the structural, optical, mechanical, thermal and electrical properties of KCl1-xBrx crystals formed directly on cooling the melt. J. Mater. Sci. 32, 15425–15440 (2021). https://doi.org/10.1007/s10854-021-06091-2
R.M. Bailey, G. Adamiec, E.J. Rhodes, OSL properties of NaCl relative to dating and dosimetry. Radiat. Meas. 32, 717–723 (2000). https://doi.org/10.1016/S1350-4487(00)00087-1
P.G. Fuochi, A. Alberti, E. Bortolin, U. Corda, S. La Civita, S. Onori, PSL study of irradiated food: NaCl as possible reference material. Radiat. Meas. 43, 483–486 (2008). https://doi.org/10.1016/j.radmeas.2007.11.015
K. Taketoshi, I. Akitoshi, Energy transfer processes from I centers to In+ centers at room temperature in co-doped NaCl:I, In+ crystals. J. Lumin. 207, 58–62 (2019). https://doi.org/10.1016/j.jlumin.2018.11.008
A. Iguchi, T. Kawai, K. Mizoguchi, Energy transfer between Tl+-type impurities in NaCl crystals. Phys. Status Solidi C 13, 85–88 (2016). https://doi.org/10.1002/pssc.201510149
M. Mehrab, M. Zahedifar, Z. Saeidi-Sogh, A. Ramazani-Moghaddam-Arani, E. Sadeghi, S. Harooni, Thermoluminescence and photoluminescence properties of NaCl:Mn, NaCL: Cu nano-particles produced using co-precipitation and sono-chemistry methods. Methods Phys. Res. Sect. A. 846, 87–93 (2017). https://doi.org/10.1016/j.nima.2016.10.001
R.B. Morgunov, M.A. Bashirov, Y.V. Malyutin, V.L. Berdinskii, Y. Tanimoto, Kinetics of transformation of Eu2+ dimers in NaCl crystals in a static magnetic field of 15 T. Phys. Solid State 49, 445–448 (2007). https://doi.org/10.1134/S1063783407030080
T. Tsuboi, H. Witzke, D.S. McClure, The 4f14→4f135d transition of Yb2+ ion in NaCl crystals. J. Lumin. 24, 305–308 (1981). https://doi.org/10.1016/0022-2313(81)90278-7
Y. Li, Y. Li, C. Li, X. Zhang, F. Zeng, H. Lin, Z. Su, Optical and mechanical properties of NaCl:Ce3+ crystal grown by the Czochralski method. J. Mater. Sci. 31, 13070–13077 (2020). https://doi.org/10.1007/s10854-020-03857-y
S.M. Levshov, I.V. Berezovskaya, N.P. Efryushina, S.I. Vdovenko, I.P. Kovalevskaya, V.P. Dotsenko, Luminescence of Eu2+ ions in alkaline earth dilithiosilicates. J. Appl. Spectrosc. 79, 70–75 (2012). https://doi.org/10.1007/s10812-012-9565-7
M.K. Hubert, Site-selective emission spectra of Eu3+:Ca5(PO4)3F. J. Alloys Compd. 302, 87–93 (2000). https://doi.org/10.1016/S0925-8388(00)00612-5
X. Huang, S. Wang, B. Li, Q. Sun, H. Guo, High-brightness and high-color purity red-emitting Ca3Lu(AlO)3(BO3)4:Eu3+ phosphors with internal quantum efficiency close to unity for near-ultraviolet-based white-light-emitting diodes. Opt. Lett. 43, 1307 (2018). https://doi.org/10.1364/OL.43.001307
H. Guo, X. Huang, Y. Zeng, Synthesis and photoluminescence properties of novel highly thermal-stable red-emitting Na3Sc2(PO4)3:Eu3+ phosphors for UV-excited white-light-emitting diodes. J. Alloy. Comp. 741, 300–306 (2018). https://doi.org/10.1016/j.jallcom.2017.12.316
Y. Li, Y. Li, F. Meng, X. Sun, X. Zhang, F. Zeng, H. Liu, Z. Su, C.K. Mahadevan, Effect of Pr ion concentration on the luminescence properties of NaCl:0.01Ce3+, Pr3+ crystals grown in large size. J. Lumin. 239, 118302 (2021). https://doi.org/10.1016/j.jlumin.2021.118302
G. Okada, S. Motoki, M.A. Sakamoto, E. Kusano, H. Nanto, Characterization of optically-stimulated luminescence prperties by NaCl:Eu2+ crystal and the thermal response. J. Alloys Compd. 863, 158561 (2021). https://doi.org/10.1143/JJAP.10.1747
I. AguirreDecarer, H.L. Dantoni, M. Barboza-Flores, V. Correcher, F. Jaque, KCl: Eu2+ as a solar UV-C radiation dosimeter OSL and TL analysis. J. Rare Earths 27, 579–583 (2009). https://doi.org/10.1016/S1002-0721(08)60292-6
J.K. Radhakrishnan, S. Selvasekarapandian, Photoluminescence of undoped and Eu doped CsCl crystals. J. Phys. 6, 6035–6041 (1994). https://doi.org/10.1088/0953-8984/6/30/021
S. Álvarez-Garcia, T.M. Piters, M. Barboza-Flores, UV induced afterglow of KCl:Eu, KBr: Eu and NaCl: Eu at low temperature. Radiat. Meas. 33, 813–817 (2001). https://doi.org/10.1016/S1350-4487(01)00188-3
H.J. Seo, W.S. Zhang, T. Tsuboi, S.H. Doh, W.G. Lee, H.D. Kang, K.W. Jang, Luminescence properties of a CsI crystal doped with Eu2+ ions. J. Alloys Compd. 344, 268–271 (2002). https://doi.org/10.1016/S0925-8388(02)00366-3
F.J. López, Optical absorption and luminescence investigations of the precipitated phases of Eu2+ in NaCl and KCl single crystals. Phys. Rev. B. 22, 6428–6439 (1981). https://doi.org/10.1103/PhysRevB.24.4847
P.V. Savchyn, V.V. Vistovskyy, A.S. Pushak, A.S. Voloshinovskii, A.I. Popov, Synchrotron radiation studies on luminescence of Eu2+ -doped LaCl3 microcrystals embedded in a NaCl matrix. Nucl. Instrum. Methods Phys. Res. B 274, 78–82 (2012). https://doi.org/10.1016/j.nimb.2011.11.024
G. Mondragon-Galicia, D. Mendoza-Anaya, M.E. Nicho-Diaz, R. Garcıa-Garcıa, J. Reyes-Gasga, Thermoluminescence response of previously heated NaCl: Eu crystals to UV radiation. J. Phys. D 41, 045103 (2008). https://doi.org/10.1088/0022-3727/41/4/045103
Y. Li, Y. Li, X. Sun, C. Li, F. Zeng, X. Zhang, J. Liu, H. Liu, Z. Su, C.K. Mahadevan, Structural, mechanical, electrical and optical properties of NaxK1-xCl:Ce3+ crystals grown in large size by the Czochralski method. Ceram. Int. 47, 34899–34908 (2021). https://doi.org/10.1016/j.ceramint.2021.09.031
Y. Li, Y. Li, F. Liu, F. Zeng, X. Zhang, D. Huang, H. Liu, J. Liu, C.K. Mahadevan, Effect of Ce concentration on the structural, mechanical, electrical and optical properties of Ce-doped large-sized KCl0.5Br0.5 crystals. J. Alloys Compd. 884, 161099 (2021). https://doi.org/10.1016/j.jallcom.2021.161099
J. Ma, X. Sun, Y. Li, X. Zhang, F. Zeng, H. Liu, C.K. Mahadevan, Study on the growth and processing technology of large-size KCl0.5Br0.5 crystal. J. Mater. Sci. 32, 16432–16444 (2021). https://doi.org/10.1007/s10854-021-06196-8
J. Nara, S. Ichi, Adachi, Optical properties of KCl:Sn2+ phosphors synthesized from aqueous KCl/SnCl2 solutions. J. Appl. Phys. 110, 113508 (2011). https://doi.org/10.1063/1.3664916
F. Clabau, X. Rocquefelte, T. Le Mercier, P. Deniard, S. Jobic, M.-H. Whangbo, Formulation of phosphorescence mechanisms in inorganic solids based on a new model of defect conglomeration. Chem. Mater. 18, 3212–3220 (2006). https://doi.org/10.1021/cm052728q
Y. Li, Y. Li, C. Li, X. Zhang, F. Zeng, H. Lin, Z. Su, C.K. Mahadevan, Structural, mechanical, thermal and optical properties of NaCl:Ce3+ single crystals grown in large size by the Czochralski method. J. Alloys Compd. 849, 156592 (2020)
J.J. Gilman, hardness of pure alkali halides. J. Appl. Phys. 44, 982–984 (1973). https://doi.org/10.1063/1.1662382
B. Subramaniam, K.G. Bansigir, Dislocation density and microhardness studies in KCl-KBr mixed crystals. J. Mater. Sci. 15, 2889–2896 (1980). https://doi.org/10.1007/BF00550560
A.K. Verma, C. Ojha, A.K. Shrivastava, Effect of impurities on the hardness of alkali halide single crystals. AIP Conf. Proc. 1242, 1591 (2014). https://doi.org/10.1063/1.4872917
S. Karan, S.P.S. Gupta, Vickers microhardness studies on solution-grown single crystals of magnesium sulphate hepta-hydrate. Mater. Sci. Eng. A 398, 198–203 (2005). https://doi.org/10.1016/j.msea.2005.03.016
J. Madhavan, S. Aruna, A. Anuradha, D. Premanand, I. VethaPotheher, K. Thamizharasan, P. Sagayaraj, Growth and characterization of a new nonlinear optical L-histidine acetate single crystals. Opt. Mater. 29, 1211–1216 (2007). https://doi.org/10.1016/j.optmat.2006.04.013
P. Sayan, J. Ulrich, Effect of various impurities on the hardness of NaCl crystals. Cryst. Res. Technol. 36, 1253–1262 (2001). https://doi.org/10.1002/1521-4079(200111)36:11%3c1253::aid-crat1253%3e3.0.co;2-2
W.A. Wooster, Physical properties and atomic arrangements in crystals. Rep. Prog. Phys. 16, 62 (1953)
F.J. López, optical absorption and luminescence investigations of the precipitated phases of Eu2+ in NaCl and KCl single crystals. Phys. Rev. B. 22, 6428–6439 (1980). https://doi.org/10.1103/PhysRevB.24.4847
A.J. Hernandez, W.K. Cory, O.J. Rubio, Optical investigation of divalent europium in the alkali chlorides and bromides. J. Chem. Phys. 72, 198–205 (1980). https://doi.org/10.1063/1.438875
I. Aguirre de Career, F. Cussó, F. Jaque, Afterglow and photoconductivity in europium-doped alkali halides. Phys. Rev. B. 38, 10812–10815 (1988). https://doi.org/10.1103/PhysRevB.38.10812
J.E. Muoz-Santiuste, J. Garcí-Solé, Precipitation-induced quenching of Eu2+ luminescence in NaCl:EuCl2. Phys. Rev. B. 38, 10874–10877 (1988). https://doi.org/10.1103/PhysRevB.38.10874
R. Cywiński, R. Fava, M. Manfredi, E. Mugeński, Aggregation processes in the NaCl:Eu2+, Mn2+ system and energy transfer mechanisms. Phys. Status Solidi 143, 433–442 (1987). https://doi.org/10.1002/pssb.2221430205
B.K. Choudhary, K.V. Rao, R.N.P. Choudhary, F-band absorption and thermoluminescence of NaCl single crystals X-ray irradiated at elevated temperatures and later subjected to high electric fields or laser excitation. J. Mater. Sci. 25, 83–86 (1990). https://doi.org/10.1016/j.jlumin.2014.04.018
R. Chen, On the calculation of activation energies and frequency factors from glow curves. J. Appl. Phys. 40, 570 (1969). https://doi.org/10.1063/1.1657437
M. Balarin, Half-width and asymmetry of glow peaks and their consistent analytical representation. J. Therm. Anal. 17, 319–332 (1979). https://doi.org/10.1007/BF01914023
S. Bangaru, D. Roobanguru, Luminescence and structural characterization on praseodymium (Pr3+) doped potassium bromide (KBr) single crystals. Luminescence 33, 885–890 (2018). https://doi.org/10.1002/bio.3486
Z.H. Ju, S.H. Zhang, X.P. Gao, X.L. Tang, W.S. Liu, Reddish orange long afterglow phosphor Ca2SnO4:Sm3+ prepared by sol-gel method. J. Alloys Compd. 509, 8082–8087 (2011). https://doi.org/10.1016/j.jallcom.2011.05.050
Acknowledgements
This work was supported by the Science and Technology Department of the Jilin Province (20200801038GH, 20200403158SF) and Changchun Science and Technology Bureau (21QC08).
Author information
Authors and Affiliations
Contributions
YL: Conceptualization, Software,Writing- Original draft preparation. XS: Data curation. SY: Visualization. HG: Resources, Validation. MM: Supervision. XZ: Writing- Reviewing. FZ: Project administration. DH: Review. CL: Formal analysis. HL: Investigation. JL: Methodology. CKM: Review & Editing.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Sun, X., Yao, S. et al. The structural, mechanical and optical properties of NaCl:Eu2+ crystal grown by the Czochralski method. J Mater Sci: Mater Electron 33, 6504–6513 (2022). https://doi.org/10.1007/s10854-022-07824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-07824-7