Abstract
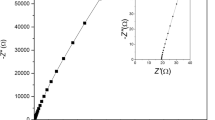
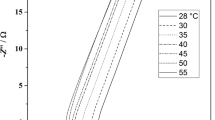
Redox capacitor, which is one type of supercapacitor, has been attracted tremendously as it shows a satisfactory specific capacitance, good cycle ability, and good stability. The present study reveals a redox capacitor fabricated with an ionic liquid (IL)-based gel polymer electrolyte (GPE). Electrodes of the redox capacitor were fabricated with the conducting polymer, polypyrrole (PPy). The composition of the GPE was polyvinylidenefluoride–co-hexafluoropropylene (PVdF–co-HFP): 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (1E3MITF): ZnTF. Characterization of redox capacitor was done by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) tests. The relaxation time constant (τ0) of the redox capacitor is about 31.57 s implying somewhat fast redox reactions. Initial single electrode specific capacitance (CSC) was 150.2 Fg−1, and at the 500th cycle, it was 40.03 Fg−1. The decrease of the CSC may be due to the formation of the passivation layer at the GPE/electrode interface, resulting in degradation upon cycling. The GCD test resulted 48.4 Fg−1 of initial single-electrode specific discharge capacitance (Csd) value. Upon 1000 cycles, it was reached 22.3 Fg−1. The decrease of Csd may be due to the degradation of the electrode and the IL-based GPE upon prolonged cycling.






Similar content being viewed by others
References
E. Frackowiak, Q. Abbas, Carbon/carbon supercapacitors. J. Energy Chem. 22, 226–240 (2013)
X. Peng, Q. Shuhai, X. Changjum, A New Supercapacitor and Li-ion battery hybrid system for electric vehicle in advisor”. J. Phys. Conf. Ser. 806, 012015 (2017)
G. Xiong, C. Meng, R. G. Reifenberger, P. P. Irazoqui, Special issue graphene a review of graphene-based electrochemical microsupercapacitors. Electroanalysis 26, 30–51 (2014)
S. Das, A. Ghosh, Solid polymer electrolyte based on pvdf-hfp and ionic liquid embedded with TiO 2 nanoparticle for electric double layer capacitor (EDLC) application. J. Electrochem. Soc. 163(13), F1348–F1353 (2017)
B. Evanko, S.W. Boettcher, S.J. Yoo, G.D. Stucky, Redox-enhanced electrochemical capacitors. ACS Energy Lett. 2, 2581–2590 (2017)
S. Uppugalla, U. Male, P. Srinivasan, Electrochimica Acta Design and synthesis of heteroatoms doped carbon/polyaniline hybrid material for high performance electrode in supercapacitor application. Electrochim. Acta 146, 242–248 (2014)
H.J. Xie, B. Gélinas, D. Rochefort, Redox-active electrolyte supercapacitors using electroactive ionic liquids Electrochemistry Communications Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. commun. 66, 42–45 (2017)
D. You, Z. Yin, Y. Ahn, S. Lee, J. Yoo, Y.S. Kim, Redox-active ionic liquid electrolyte with multi energy storage mechanism for high energy density. RSC Adv. 7, 55702–55708 (2017)
G.P. Pandey, S.A. Hashmi, Ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate-based gel polymer electrolyte for electrochemical capacitors. J. Mater. Chem. A 1(10), 3372 (2013)
C.W. Liew, Y.S. Ong, J.Y. Lim, C.S. Lim, K.H. Teoh, S. Ramesh, Effect Of Ionic Liquid On Semi – crystalline Poly ( vinylidene fluoride – co – hexafluoropropylene ) Solid Copolymer Electrolytes. Int. J. Electrochem. Sci. 8, 7779–7794 (2013)
J. Bai, H. Lu, Y. Cao, X. Li, J. Wang, A novel ionic liquid polymer electrolyte for quasi-solid state lithium air batteries. RSC Adv. 7(49), 30603–30609 (2017)
C. Liew, K.H. Ari, J. Kawamura, Y. Iwai, S. Ramesh, A.K. Arof, Effect of halide anions in ionic liquid added poly ( vinyl alcohol ) -based ion conductors for electrical double layer capacitors. J. Non. Cryst. Solids 458, 97–106 (2017)
A. Brandt, S. Pohlmann, A. Varzi, A. Balducci, S. Passerini, Ionic liquids in supercapacitors. MRS Bull. 38, 554–559 (2013)
G.P. Pandey, Y. Kumar, S.A, Hashmi, Ionic liquid incorporated polymer electrolytes for supercapacitor application. Indian J. Chem. 49(5–6), 743–751 (2010)
M. Moreno et al., Ionic liquid electrolytes for safer lithium batteries I. investigation around optimal formulation. J. Electrochem. Soc. 164(1), A6026–A6031 (2017)
M. Armand, F. Endres, D.R. MacFarlane, H. Ohno, B. Scrosati, Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 8(8), 621–629 (2009)
J. Chattoraj, D. Diddens, A. Heuer, Effects of ionic liquids on cation dynamics in amorphous polyethylene oxide electrolytes. J. Chem. Phys. 140(2), 2–8 (2014)
S.P. Ong, O. Andreussi, Y. Wu, N. Marzari, G. Ceder, Electrochemical windows of room-temperature ionic liquids from molecular dynamics and density functional theory calculations. Chem. Mater. 23(11), 2979–2986 (2011)
B.A. Mei, O. Munteshari, J. Lau, B. Dunn, L. Pilon, Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 122(1), 194–206 (2018)
H. Yu et al., A novel redox-mediated gel polymer electrolyte for high-performance supercapacitor. J. Power Sour. 198, 402–407 (2012)
J.P. Tey, M.A. Careem, M.A. Yarmo, A.K. Arof, Durian shell-based activated carbon electrode for EDLCs. Ionics (Kiel) 22(7), 1209–1216 (2016)
R. Ramya, R. Sivasubramanian, M.V. Sangaranarayanan, Conducting polymers-based electrochemical supercapacitors — Progress and prospects. Electrochim. Acta 101, 109–129 (2013)
C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang, J. Zhang, A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 44(21), 7431–7920 (2015)
N. Harankahawa, S. Weerasinghe, K. Vidanapathirana, K. Perera, Investigation of a Pseudo Capacitor with Polyacrylonitrile based Gel Polymer Electrolyte. J. Electrochem. Sci. Technol. 8(2), 107–114 (2017)
P.A. Basnayaka et al., High Performance Asymmetric Supercapacitors Based on Dual Phosphorus ( P ) and Nitrogen ( N ) co-Doped Carbon and Graphene-Polyaniline Electrodes. J. Solid State Sci. Technol. 6(6), 3168–3172 (2017)
G.P. Pandey, S.A. Hashmi, Y. Kumar, Performance studies of activated charcoal based electrical double layer capacitors with ionic liquid gel polymer electrolytes. Energy Fuels 24(12), 6644–6652 (2010)
A. Rezqita, M. Sauer, A. Foelske, H. Kronberger, A. Trifonova, The effect of electrolyte additives on electrochemical performance of silicon/mesoporous carbon (Si/MC) for anode materials for lithium-ion batteries. Electrochim. Acta 247, 600–609 (2017)
A. Gupta, S.K. Tripathi, Effect of anionic size of PMMA Based Polymer Gel Electrolytes for Redox Capacitor. Int. J. Eng. Res. Appl. 3(1), 3–5 (2013)
M. Mastragostino, C. Arbizzani, F. Soavi, Polymer-based supercapacitors. J. Pow. Sour. 97–98, 812–815 (2001)
A. Jain, S.K. Tripathi, Experimental studies on high-performance supercapacitor based on nanogel polymer electrolyte with treated activated charcoal. Ionics (Kiel) 19, 549–557 (2013)
K. Wang, H. Wu, Y. Meng, Y. Zhang, Z. Wei, Integrated energy storage and electrochromic function in one flexible device: an energy storage smart window. Electron. Suppl. Mater. Energy Environ. Sci. 5(8), 8384 (2012). https://doi.org/10.1039/c2ee21643d
K.S. Ryu, K.M. Kim, N. Park, Y.J. Park, S.H. Chang, Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Pow. Sour. 103(2), 305–309 (2002)
S. Palaniappan, S.B. Sydulu, T.L. Prasanna, P. Srinivas, High-temperature oxidation of aniline to highly ordered polyaniline – sulfate salt with a nanofiber morphology and its use as electrode materials in symmetric supercapacitors. J. Appl. Polym. Sci. 120, 780–788 (2011)
F. Ataherian, N. Wu, Long-term charge/discharge cycling stability of MnO 2 aqueous supercapacitor under positive polarization. J. Electrochem. Soc. 158(4), A422–A427 (2011)
Acknowledgements
Authors wish to acknowledge National Science Foundation Sri Lanka for the financial support under the grant, RG/2017/BS/02 and Wayamba University of Sri Lanka. Kuliyapitiya, Sri Lanka.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prasadini, K.W., Perera, K.S. & Vidanapathirana, K.P. Preliminary study on the performance of a redox capacitor with the use of ionic liquid-based gel polymer electrolyte and polypyrrole electrodes. J Mater Sci: Mater Electron 32, 17629–17636 (2021). https://doi.org/10.1007/s10854-021-06296-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06296-5