Abstract
In low- to middle-income communities, the lack of affordability of conventional sanitary products during menstrual cycles can cause psychological and health issues, ultimately affecting their quality of life. It is crucial to develop alternative products that are affordable and accessible to all while also promoting menstrual health and hygiene. Super absorbent polymers (SAPs) are a vital component in current disposable sanitary pads and nappies. However, these SAPs are often non-biodegradable and non-biocompatible. Therefore, the use of eco-friendly materials for the production of SAPs is gaining popularity in the hygiene industry, as it offers a means to reduce the carbon footprint and environmental impact associated with traditional SAPs made from non-renewable petroleum-based materials. SAPs made from polysaccharides often have naturally occurring antibacterial properties, making them appealing for commercial applications in sanitary products such as sanitary pads. In addition, the move toward reusable sanitary pads with antibacterial properties can significantly reduce waste generated by single-use products and prevent the growth of bacteria, improving the safety and hygiene of the product. Furthermore, computational modeling and artificial intelligence are now important tools in SAP synthesis, providing advantages such as predicting polymer properties, rationalizing synthesis pathways, and improving quality control. These tools can reduce synthesis costs by eliminating the need for trial-and-error approaches in polymer synthesis, ultimately promoting more affordable products for end users. Overall, these advancements in polymer synthesis and material design can help to create a more sustainable industry and promote menstrual hygiene and product accessibility to those who need it most.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acknowledging and prioritizing the sensitive psychosocial, health, and hygiene continuum of menstrual circumstances is gaining international policy importance in a bid to promote dignity, gender equality, and reproductive health among all groups but especially among vulnerable and lower-income groups [1]. In 2012, the formalization of MHM (Menstrual Health Management) acknowledged challenges associated with menstruation, emphasizing privacy and changing of sanitary materials, access to water, sanitation, and hygiene (WASH) facilities, safe disposal areas, and the importance of comprehensive menstrual cycle knowledge with dignity. All of this should also be done with relevant and correct knowledge about the menstrual cycle and how to manage it without discomfort or fear, but with dignity [2]. There is consistent evidence of wealth-related inequality and “period poverty” in the menstrual continuum: Lack of education and poor literacy, access, usage, and disposal of pads and even infrastructural limitations of households all contribute to such imbalances [3]. Limited awareness, bias, cultural norms, resource constraints, environment, safety concerns, and product availability hinder thorough menstrual product testing and personalized management [4]. Although most of the research on menstrual hygiene management focuses on the challenges faced by women in sub-Saharan Africa and South Asia [5], similar issues persist in other regions, including East Asia, Latin America, the Caribbean, and the Middle East [6]. The high cost of reliable sanitary products adversely affects education, gender equality, and women's reproductive health globally [7]. The high cost of dependable sanitary products can result in school absenteeism, hindering education attainment and gender equality for girls in low-income areas [7]. Since traditional methods of controlling menstrual bleeding have unreliable levels of absorbency and are unsanitary and dangerous to women’s reproductive health, the availability of high-quality, absorbent, and affordable menstrual hygiene products is urgently needed.
Disposable sanitary pads (DSPs) are single-use products used during menstrual periods. However, the disposal of sanitary products is a significant challenge, particularly the plastic-lined ones, which can contain harmful substances, posing environmental and health risks. It has been reported that DSPs estimated annual solid waste could amount to 44 254 cm3 per user per year [8]. In context of global efforts aimed at reducing single-use plastic, the conventional utilization of plastic-laden disposable service products present a challenge owing to their slow decomposition rate (500–800 years [9]), thereby causing detrimental effects on the environment. Improper disposal, highlighted in a review on menstrual hygiene management in India and South Africa, [10] exacerbates waste management issues, with alarming findings such as 20 sanitary items per 100 m of shoreline in 2016 and an estimated 9000 tons of sanitary waste annually in India alone [11]. DSPs typically have a hydrophobic outer layer with unique patterns (holes) that lead menstrual fluids away from the body, where various inner layers absorb them. The back layer consists of hydrophobic plastics to prevent leakage and the inner layer is typically comprised of an absorbent layer, typically a superabsorbent polymer. In plastics, chemicals like phthalates are present and they are used as plasticizers in various industrial and consumer products (e.g., in the top layer and plastic of DSP). Epidemiologic studies have linked exposure to phthalates with precocious puberty, endometriosis, female genital tumors, and ovulation disorders [12].
Superabsorbent polymers (SAPs) are synthetic materials made from slightly cross-linked polyacrylic acid, polyacryl amides, polyacrylonitriles, and their salt derivatives [13]. These polymers have the ability to absorb and retain large amounts of water or aqueous solutions, up to several hundred times their own weight. This property makes them useful in a wide range of industries, sanitary products, wastewater treatment, construction and agriculture [14,15,16,17]. SAPs are produced by polymerizing acrylic acid or one of its derivatives, such as sodium acrylate or potassium acrylate, which are then cross-linked with a chemical cross-linker, forming a three-dimensional network of polymer chains [13]. One of the advantages of polyacrylic-based SAPs is their cost-effectiveness, as the raw materials are readily available and the production process is relatively straightforward. Additionally, these polymers have good biocompatibility and are generally safe for use in medical and personal care products. However, SAPs are not without limitations. They can release small amounts of residual monomers or cross-linkers during use, which can be a concern in some applications [19]. Furthermore, the disposal of SAP-containing products can be problematic, as they do not readily biodegrade and can contribute to environmental pollution. Petroleum-based SAPs pose a challenge due to their complex cross-linked structures and high molecular weight, which results in poor biodegradability [13]. Additionally, the raw materials necessary for synthesizing most SAPs come from fossil fuels, rendering them non-renewable.
Consideration of key factors is crucial when designing a sanitary pad that prioritizes safety and comfort. These essential points are outlined in Table 1, detailing the criteria for creating an ideal sanitary pad. Meeting these criteria ensures that modern sanitary pads are not only effective in managing menstrual hygiene but also prioritize comfort, safety, and environmental sustainability.
This review addresses limitations in SAPs and highlights a literature gap on nanotechnology’s role in providing self-cleaning and antibacterial/fungal properties to reusable sanitary napkins. This is important because since proper menstrual hygiene is crucial for minimizing infection risks, cervical cancer, and rashes during menstruation.
Types of SAPs
Super absorbing polymers (SAPs) are hydrophilic materials with high absorption capacities for water and aqueous liquids relative to their mass, thus making them attractive for commercial applications in agriculture, wound dressing, construction, hygiene, etc. In agriculture, they are used as controlled delivery systems of agrochemicals, pesticide carriers, and to maintain soil humidity by improving water retention capacity [20,21,22,23]. The incorporated SAPs reduce drought stress and prolong the time needed before the next irrigation cycle, ultimately reducing water wastage and optimizing profits [24]. The construction industry uses SAPs to enhance the multiple properties of concrete, such as preventing crack formation, fire-retardation, self-healing concrete, and controlling water absorption and release [25, 26]. In the electronics space, SAPs are investigated as electrolytes in flexible Zn-based batteries [27, 28]. In the hygiene and cosmetics industries, SAPs are used in sanitary pads, diapers, and as biocosmetics (100% natural ingredients) [29]. SAPs also have many applications in medicine, ranging from drug delivery systems to wound healing and even disposable medical protective clothing [30,31,32]. In disposable medical protective clothing, SAPs contribute to fluid management, odor, and infection control which are needs in healthcare settings especially during medical operations.
SAPs can be categorized according to the type of raw material used to prepare them, synthetic (petroleum based) and natural (polypeptide and polysaccharide based). SAPs are further classified as physical absorbers because of their mechanism of water absorption, which includes (1) entrapment of solution molecules by capillary forces, (2) changes of the crystal structure, (3) a combination of the two, or (4) coupled combination of one and two with the dissolution and expansion of the cross-linked polymer chains. Typically, absorption and expansion of the SAP is dependent on the amount of cross-linkages present. Compared to high density cross-linked SAPs, low density cross-linked SAPs exhibit better expansion and absorbent capacity [24]. The polymer chains contain ions that induce an osmotic gradient through which water is absorbed with hydrogen bonding the primary mode for holding the water molecules within the polymer chains [24, 33].
Petroleum-based SAPs
Polyacrylate-based SAPs are the most widely utilized, primarily due to their low-cost and exceptional ability to absorb water. These SAPs are presently produced from petroleum-based feedstock through the polymerization/copolymerization of acrylic acid, sodium acrylate, acrylamide, and/or acrylonitrile. Due to cross-linking (which creates a 3D structure), these polyacrylate SAPs are stable and non-biodegradable. Even though a bio-based method can partially produce acrylic acid, the disadvantage is that the resulting acrylate-based SAPs have limited biodegradability [34,35,36,37].
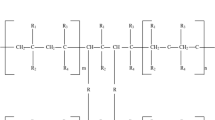
Sodium polyacrylate is a crucial component in sanitary products such as diapers and sanitary pads. It has an absorption capacity hundreds of times its weight and exceptional water retention capabilities even when subjected to pressure [38]. The mineral content of solutions influences the osmotic flow required for aqueous solution absorption; thus, deionized water has the highest absorption capacity, followed by tap water and saline solutions. Because blood and urine contain mineral salts such as NaCl, this should be considered when developing SAPs for sanitary products. Water absorption capacity, defined as the weight of absorbed liquid divided by the dry weight of SAP, is a fundamental parameter that describes SAPs' ability to retain water [13]. Polyacrylamide is another type of SAP that possesses non-toxic properties and can be broken down by microorganisms through aerobic processes, rendering it an eco-friendly option [39,40,41]. However, the starting monomer acrylamide is petroleum based and is considered a carcinogenic agent [42, 43] (Fig. 1).
Illustration of the preparation processes of a modified PVA fibers and b their implementation as hygroscopic and strong antistatic medical protective clothing material [30]. Reprinted from Adv. Fiber Mater., 2, Yang, L.; Liu, H.; Ding, S.; Wu, J.; Zhang, Y.; Wang, Z.; Wei, L.; Tian, M.; Tao, G., Superabsorbent Fibers for Comfortable Disposable Medical Protective Clothing, 140–149, Copyright (2020), with permission from Springer Nature.
Several reports are available on the synthesis and structural modifications of SAPs through surface cross-linking techniques to improve their water absorption properties. For instance, Azizi and colleagues employed polyamine modifiers such as diethylenetriamine (DETA) and polyethyleneimine (PEI) to modify the surface of terpolymers, with or without using an AlCl3 catalyst. When DETA was used as the surface modifier in free swelling measurements, the addition of the catalyst decreased the absorption capacity in both distilled and saline water. However, when absorption under load (AUL) measurements were conducted, absorption increased from 17.71 ± 0.71 to 23.46 ± 0.94 g/g. Similarly, when PEI was used as the surface modifier, the AUL also increased from 17.71 ± 0.71 to 24.82 ± 0.94 g/g with the addition of the catalyst. Consequently, it was concluded that adding surface modifiers and an AlCl3 catalyst enhanced absorption under load by approximately 25% [44]. Another study involved cross-linking the surface region of SAP spheres with ethylene glycol diglycidyl ether to improve their mechanical properties [45, 46]. Enhancing the surface contact between the SAPs and water accelerates the water absorption. This is achieved by increasing the specific surface area of SAPs, which can be done through the creation of a porous structure (see Fig. 2) [47]. There are various methods to create the porous structure of SAPs, such as phase inversion [48,49,50], freeze-drying and hydration [51,52,53,54], water-soluble porogens [55,56,57,58], and foaming [59,60,61]. Researchers have also investigated different drying techniques, such as using water vapor from the dehydration of Al(OH)3, to create porous SAPs [62].
Scanning electron micrographs of a non-porous SAP (A) and a corresponding SPH (B and C) [47]. Reprinted from J. Control. Release., 102, Omidian, H.; Rocca, J. G.; Park, K., Advances in Superporous Hydrogels, 3 -12, Copyright (2005), with permission from Elsevier.
The problem with petroleum-derived SAPs is that their complex cross-linked structures and high molecular weight afford low biodegradability [13]. Moreover, the raw materials required for synthesizing most SAPs are fossil fuels, thus making them non-renewable. Firstly, there is a high demand for fossil fuels to support a rapidly growing population, which has resulted in their overconsumption and will soon be depleted. To promote sustainable development, shifting toward SAPs made from renewable resources is necessary. Developing more environmentally friendly and biodegradable SAPs can effectively address these limitations (Fig. 3).
Biopolymers
The use of SAPs as the primary materials for commercial sanitary pads became popular in Japan and the USA in the 1970s [63, 64]. The high production cost and significant environmental impact of synthetic polymers have been two major concerns regarding the production of SAPs, despite their superior absorption capacity [65,66,67]. In comparison, natural plant fibers like cellulose are highly absorbent due to their ability to attract water. The cell walls of these fibers contain hydroxyl and other oxygen-containing groups that attract moisture through hydrogen bonding, causing the fibers to expand as they absorb water. Cotton fibers can absorb up to 24–27 times their weight [68]. Although natural fiber does not have the absorption capability of SAPs, there are examples of SAPs prepared from natural feedstock with good absorption capability [69,70,71,72]. Superabsorbent polymers made from natural products have recently received much attention due to their positive environmental impact [73,74,75,76,77,78,79,80]. These SAPs are primarily composed of polysaccharides, and their primary advantage is their biodegradability, low-cost synthesis, and endorsement of a circular economy (see Fig. 4 for some common polysaccharides). Biodegradable polymers can degrade under ambient conditions by chemical hydrolysis or through enzymatic actions of microorganisms.
Top: The schematic representation of the synthesis of carboxymethylcellulose/polyacrylamide composite. Bottom Left: The SEM image of carboxymethylcellulose. Bottom Right: The SEM image of the carboxymethylcellulose/polyacrylamide composite. Reprinted from J. Hazard. Mater., 364, Godiya, C. B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X., Carboxymethyl Cellulose/Polyacrylamide Composite Hydrogel for Cascaded Treatment/Reuse of Heavy Metal Ions in Wastewater, 28–38, Copyright (2019), with permission from Elsevier.
Polysaccharides are abundant with hydroxyl, carboxyl, and amine groups that can be used to prepare superabsorbent polymers with fascinating structures and properties. Many natural SAPs have been synthesized from alginate, cellulose, chitosan, and starch [73]. Natural SAPs are more attractive than their petroleum-based counterparts in personal hygiene products, because of their biocompatibility and as a result, they have no harmful or toxic effects on human skin [14]. Therefore, bio-SAPs with exceptionally high absorption capacities must be developed to compete commercially with the existing acrylate-based polymers. The high functionalization capabilities of polysaccharides make them ideal candidates as SAPs due to their ability to produce SAPs with impressive absorption and retention capacities for aqueous solutions.
Cellulose is the primary component of plants, and it can form 3D networks of superabsorbent polymers [14]. It is biodegradable, biocompatible, highly abundant, inexpensive, and originates from a renewable resource. SAPs produced from cellulose are insoluble in water, although they can absorb large amounts of water and aqueous solutions. Cellulose-based SAPs can also be prepared by applying cryogenic treatment at ambient temperature. These SAPs (cryogels) can be formed by hydrogen bonding during one of the stages of the freeze–thawing cycle. Repeating the freeze–thawing cycles introduces physical cross-linking and crystallinity [81,82,83,84]. With this background, Zhang and colleagues reported a series of polyvinyl alcohol (PVA) and sodium carboxymethylcellulose (CMC) hydrogels synthesized by physical mixing and freeze–thawing [85]. The interaction between the two molecules resulted in intramolecular entanglements forming three-dimensional polymeric physical cryogels, which are stabilized by the nature of these bonds. During freeze–thawing, the polymer chains create ordered structures called microcrystalline zones that act as junction knots of the network. Most polysaccharides contain hydroxyl and carboxyl groups; hence, it is suggested that the intermolecular forces in these zones are hydrogen bonding. Kim and colleagues report a crystalline bacterial cellulose (BC) and PVA composite hydrogel with enhanced mechanical strength. Subjecting the hydrogel to repetitive freeze–thaw cycles resulted in the spontaneous crystallization of polymer chains around the BC nanofibers, ultimately increasing the mechanical strength of the gel [84]. This methodology is particularly promising for developing robust hydrogels since it enhances mechanical strength without the need for chemical cross-linkers and additives. Kumar–Seera also synthesized microcrystalline hydrogels that were copolymerized with cellulose and polyvinyl alcohol using both physical and chemical cross-linking techniques [83]. They report that physical cross-linking was achieved through freeze–thawing, whereas chemical cross-linking was ensued by ethylene glycol diglycidyl ether as a cross-linker.
Starch is a frequently occurring biodegradable polymer comprising of a mixture of amylose and amylopectin. Its popularity stems from its low-cost, biocompatibility, straightforward production, abundant availability, non-toxicity, and biodegradable nature [86]. However, unmodified starch has poor thermal and mechanical characteristics and it easily absorbs water (which is a good characteristic for use in SAPs, but not for other uses), making it unsuitable for direct use [87]. To address these issues, starch must undergo genetic, enzymatic, physical, and chemical modifications. Depending on the application, starch/carboxymethylcellulose SAPs may offer a low-cost and superior alternative to pure cellulose derivative-based gels. Another method used to introduce physical cross-linking is electron beam irradiation [88, 89]. Over the years, starch/carboxymethylcellulose SAPs have been synthesized through gamma irradiation in aqueous mixtures of starch and carboxymethylcellulose [90,91,92,93,94]. Fekete and colleagues synthesized a superabsorbent polymer using gamma irradiation, with starch partially replacing carboxymethyl cellulose (CMC). Substituting CMC with starch enhanced the gel fraction and water uptake, but excessive amounts of starch had a detrimental effect on gelation. The optimal hydrogel composition contained 30% starch, exhibiting excellent properties such as a water uptake of approximately 350 g/g. Research by Czarnecka and Nowaczyk resulted in the production of starch hydrogels cross-linked with acrylic monomers. For this series of SAPs, absorption capacity was represented in gel percentage (gel %). Maximum absorption for the SAPs varied between 18989–1547 gel % for deionized water and between 1047 and 2165 gel % for the saline solution [73]. Dispat and colleagues [95] synthesized a superabsorbent polymer for agricultural application using modified starch. The starch was modified using zinc oxide and tetraethyl orthosilicate, then grafted with potassium acrylate monomer, resulting in improved reusability, biodegradability, soil conditioning properties, and resistance to transient drought. Using gamma irradiation, SAPs were prepared from aqueous solutions of four cellulose derivatives, including carboxymethylcellulose, methylcellulose, hydroxyethyl cellulose, and hydroxypropyl cellulose, using N-methylene-bis-acrylamide as a cross-linker. The swelling rate was highest in the first 5–6 h, then gradually decreased. Carboxymethylcellulose gels without a cross-linker reached equilibrium water uptake after 24 h, while in the presence of a cross-linker, the water uptake almost reached equilibrium after 6–10 h [96]. Pourjavadi and colleagues observed pH-reversible behavior when they grafted polyacrylonitrile onto carboxymethyl cellulose using ceric ammonium nitrate as an initiator for free-radical polymerization [97].
Chitin is the second most abundant polysaccharide in plant-based fungi and crustaceans [98,99,100]. Chitosan is a derivative of chitin and is formed from the deacetylation of the N-acetyl group of chitin, ultimately obtaining free amine side groups (see Scheme 1) [98, 101]. When the degree of deacetylation is greater than 50%, the chitin derivative is referred to as chitosan [105]. Cheng and colleagues have reported that complete deacetylation of chitin to chitosan with microwave and conventional heating methods is impossible [102]. On the contrary, Sivashankari and colleagues reported 100% chitin deacetylation in 50 w/w% sodium hydroxide for 48 h at 100 °C and 82% deacetylation when the reaction was left for one hour [103]. Additionally, a longer reaction time results in chain degradation of the chitosan-chitin, as indicated by the lower molecular weight of the product after 48 h reaction time. Both chitin and chitosan may be modified and used as raw materials to obtain biodegradable, biocompatible, low-cost biopolymers for use in, e.g., SAPs. Using pure chitosan in SAP synthesis favors both human health and the environment. Chitosan is derived from a renewable source and has antibacterial properties [104,105,106,107]. Although biopolymers like chitosan have low ductility, they possess superior mechanical and degradation properties. A lower degree of deacetylation of chitosan leads to better mechanical properties. Heating an aqueous chitosan solution above its critical temperature (42.5 °C) can create an elastic and thermo-reversible gel [108]. Higher concentrations of chitosan require lower temperatures for gelation. This gelation process destroys the solvated chitosan structure, which exposes hydrophobic regions and leads to the formation of hydrophobic aggregates. A chitosan starch citrate cross-linked polymer demonstrated a high capacity for absorbing water in both aqueous and saline environments [98]. The presence of amino (–NH2) and carboxyl (–COOH) groups in the chitosan backbone makes it a potential candidate for the synthesis of SAP. These functional groups in the structure make it easy to perform graft polymerization of hydrophilic vinyl monomer chains onto the chitosan, resulting in the efficient synthesis of biodegradable SAPs/hydrogels. Chitosan SAPs can be produced via coagulation in an alkaline medium, which reduces the repulsion of the –NH3+ functionalities on the different chitosan chains [109, 110]. Adding the hydrophobic cross-linker β-glycerophosphate during chitosan hydrogel formation creates a favorable environment for gel structure formation [111, 112]. This results in increased pH and ionic strength of the chitosan solution, which reduces electrostatic repulsion and enhances polymer–polymer hydrophobic interactions.

Adapted from Macromol. Res., 23, Park, J. P.; Koh, M.-Y.; Sung, P. S.; Kim, K.; Kim, M. S.; Lee, M. S.; Shin, E.-C.; Kim, K. H.; Lee, H., Inactivation Efficiency of DNA and RNA Viruses during Chitin-to-Chitosan Conversion, 505–508.
General schematic diagram for the deacetylation of chitin N-acetyl groups to chitosan [113].
Superabsorbent hydrogels prepared from cassava starch, acrylic acid, and chitosan/cellulose nanocomposites exhibited improved swelling capacities [114]. The maximal swelling capacity of these polysaccharide-based hydrogels reached 767 g/g in urea solution, compared to the dry weight [115]. The degree of cross-linking affects both the swelling capacity and absorption rate of these hydrogels, with a high cross-linking percentage making the polymer more rigid but less prone to swelling [73]. The presence of hydrophilic chains in the hydrogel's backbone and the ionization of carboxyl groups at higher pHs contribute to the improved water absorbency of these hydrogels. A superabsorbent hydrogel made from chitosan-graft-poly(acrylic acid) [CT-PAA] and rice husk ash (RHA) was synthesized and studied for its swelling behavior in solutions of varying pH (2–12). The swelling and the deswelling response were highly sensitive to the pH of the solution [116]. As the pH level rose from 2.0 to 8.0, the hydrogels displayed a rise in water absorbency. However, water absorbency began to decline beyond a pH level of 8.0. At an optimal pH level of 8.0, the water uptake for CTS-graft-PAA/RHA calcined at 900 \(^\circ{\rm C}\) and CTS-graft-PAA were observed to be 64 g/g and 46 g/g, respectively. The difference in water uptake between the two hydrogels indicates incorporating RHA into the CTA-PAA enhanced the hydrogel’s absorption property. Furthermore, modifying chitosan-based superabsorbent hydrogel by adding hydrophilic polyacrylamide chains resulted in a higher water retention capacity and swelling percentages of 1897.2%, 1507.1%, and 1432.2% after 50 h [117].
A new superabsorbent composite with exceptional swelling capabilities was created through graft copolymerization of partially neutralized acrylic acid onto a sodium alginate backbone in the presence of organo-loess [118]. Loess is a naturally occurring mineral consisting of finely ground dust or silt. It is created by glaciers grinding rocks into fine powder or by wind carrying dust particles [119,120,121]. Over time, this sediment forms an inorganic mineral composed of carbonates and silicates, which contain reactive groups on its surface. Loess has hydrophilic properties that can enhance network structure and absorptive capacity [118]. The composite's water absorption capacity was remarkable, with a maximum equilibrium absorbency of 656 g/g in distilled water and 69 g/g in a 0.9 wt% NaCl solution. Additionally, the superabsorbent composite displayed excellent pH buffer ability in the range of 4 to 10 and could retain water effectively. However, the current production of bio-based products, which primarily relies on first-generation feedstocks such as corn, sugarcane, and rapeseed, poses ethical and environmental concerns by competing with the food and feed industry. Hence, much effort is being put into using lignocellulosic feedstocks (non-food crops or second-generation), which is a more attractive option as it is the most abundant and renewable resource on earth.
A novel SAP was created by cross-linking lignosulfonate, sodium alginate, and konjaku flour, which exhibited a maximum water absorption capacity of 41.23 g/g [122]. A lignin-based SAP is produced using lignin alkali polymers and poly(ethylene glycol) diglycidyl ether in an alkali solution [123]. The SAP has a water swelling capacity of 34 g/g dry weight in distilled water. Biodegradability and phytotoxicity tests reveal that 6.5% of the SAP mass decomposed after 40 days of incubation in a soil solution. Lignin-PVA SAPs are prepared from lignin, PVA, and epichlorohydrin as the cross-linker and these SAPs can swell up to 456 g/g under mild conditions [124].
Hybrid polymers
Cross-linked petroleum-based polyacrylate and polyacrylamide SAPs demonstrate the highest absorption capacity, while polyacrylamides also display some degree of salt resistance due to their nonionic nature [13, 125]. As a result, these synthetic SAPs are still highly favored for commercial applications, but their drawback is that they are non-biodegradable and sometimes non-biocompatible. Naturally generated SAPs based on polysaccharides are more environmentally friendly owing to their better biodegradability and biocompatibility, but they have low water absorption capacities compared to petroleum SAPs. Therefore, hybrid SAPs comprised of a natural polysaccharide and a synthetic monomer have recently gained attention as a potential mitigation strategy for the above-mentioned shortfalls of both synthetic and natural SAPs [126, 127].
There have been successful reports in the water purification industry for the application of hybrid SAPs as absorbents. Godiya and colleagues synthesized a hybrid carboxymethyl cellulose (CMC) cross-linked with a polyacrylamide (PAM) monomer for the absorption of heavy metals for wastewater treatment (Fig. 4) [128]. The hybrid SAP displayed an affinity for divalent copper, lead, and cadmium ions. The absorption process at equilibrium was attributed to both the Langmuir adsorption model and pseudo-second-order kinetics. The absorbed copper ions were reduced and then successfully applied as catalysts for the reduction of 4-nitrophenol to 4-aminophenol. Some hybrids consist of an inorganic–polysaccharide mixture, and the inorganic functionality is typically silica (SiO2) [129, 130]. Panao and colleagues synthesized hybrid SAPs composed of an alginate polysaccharide cross-linked with polyacrylate and polyacrylamide monomers [129]. SiO2 microspheres were π-bonded to the SAPs, and these SAPs exhibit exceptional water absorption capabilities of 889.76 ± (12.65) per gram of dry polymer. The superabsorbent properties of the polymers are primarily influenced by the surface charge of the microspheres and the chemical composition of the polymer, which collectively contribute to the creation of a highly hydrophilic structure. The SAPs were applied in methylene blue absorption. At equilibrium, the adsorption process was correlated to the Langmuir model, suggesting chemisorption due to electrostatic interactions being the primary mode of absorption. Chen and colleagues reported a hybrid SAP with polyacrylic acid and silica nanoparticles with a superior water absorption capacity of 5000 g/g in deionized water, which is admirable as most SAPs do not absorb more than 3000 times their weight [130]. Other composites comprise of organically produced monomers such as citric acid and itaconic acid [131, 132].
Modes of SAP formation
Effective absorption of aqueous solutions requires a highly cross-linked network. Either physical or chemical methods can achieve this cross-linking. Physical methods include ionic interactions, hydrogen bonding, hydrophobic bonding, and the entanglement of polymer chains without a chemical cross-linker [14, 127]. The absence of cross-linkers in physical cross-linking methods maintains the natural polymer’s low toxicity and biocompatibility, thereby preserving their benefits. However, the drawback of physical methods is that they produce SAPs whose cross-linking is reversible, thus making them unstable, whereas chemical cross-linking is permanent [127]. Therefore, chemical cross-linking is the preferred alternative due to the production of stable and resistant polymers. Physical cross-linking involves physical interaction between monomers and the polymer macromolecule, and chemical cross-linking involves the formation of new covalent bonds [127]. Chemical cross-linking is typically categorized into: solution radical graft polymerization, inverse suspension polymerization, microwave polymerization, and non-radical polymerization [14, 127, 133].
Solution radical graft polymerization
The most widely used method for chemical cross-linking involves free-radical graft polymerization (see Scheme 2), which takes place in an aqueous medium, and the SAPs are prepared from four main components: (1) polysaccharide macromolecule, (2) monomer, (3) initiator, and (4) a cross-linker [134]. During this process, the initiator is exposed to heat or radiation and decomposes to form free radicals [133, 135]. The radicals further react with water to form hydroxyl radicals. These hydroxyl radicals extract hydrogen atoms from the functional groups (hydroxyl, carboxyl) on the polysaccharide macromolecule forming reactive oxygen atoms. Ultimately the initiator creates reactive free radicals on the macromolecule and monomer that lead to grafting [133]. Cross-linking is then initiated by a chemical cross-linker such as N,N′-methylenebisacrylamide (MBA). Cross-linking can also occur without using a chemical cross-linker, depending on the type of polysaccharide and its functional groups [14, 135]. Upon addition of the initiator, the reaction generally increases, and this temperature elevation facilitates the creation of active sites on the monomers and macromolecule backbone [134,135,136].
General schematic representation for superabsorbent polymer formation using cellulose as the reference polysaccharide [14, 135]. Reprinted from Waste Biomass Valor., 11, Abou-Baker, N. H.; Ouis, M.; Abd-Eladl, M.; Ibrahim, M. M., Transformation of Lignocellulosic Biomass to Cellulose-Based Hydrogel and Agriglass to Improve Beans Yield, 3537–3551, Copyright (2020), with permission from Springer Nature.
Inverse-phase suspension polymerization
Similar to radical graft polymerization, the process of inverse-phase suspension polymerization is comprised of a (1) polysaccharide macromolecule, (2) initiator, (3) monomer, and a (3) cross-linker [137]. However, in graft polymerization, the reaction occurs solely in an aqueous medium [14, 133, 134]. Inverse-phase suspension polymerization is a type of polymerization process that involves the formation of polymer particles in an immiscible liquid phase, which is dispersed as droplets in a continuous organic phase [14, 138]. Typically, in this process the monomer, cross-linking agent, and the initiator are dissolved in the aqueous phase and the surfactant is dissolved in the organic phase. The aqueous phase is continuously to the organic phase containing the surfactant (stabilizer) which prevents the droplets' coalescence and leads to a stable suspension [14, 139].
During the polymerization reaction, the monomer molecules diffuse from the non-aqueous phase into the aqueous phase and react with the initiator to form polymer chains. As the polymerization proceeds, the polymer chains grow and eventually form solid polymer particles within the non-aqueous droplets [140,141,142,143]. Inverse-phase suspension polymerization has several advantages over other polymerization processes, such as high reaction rates, good control of morphology particle size, and the ability to produce particles with a wide range of compositions and properties [144]. It is commonly used for the production of polymer particles for applications such as coatings, adhesives, and biomedical materials. One disadvantage is that the removal of surfactants and organic solvents from SAPs can be quite difficult, requiring extensive washing and, in some cases, purification through heat application [14, 144,145,146]. If not adequately removed, they may be toxic, thus rendering the “biocompatibility” of the polysaccharide polymer null and void. Furthermore, research done by Sand and colleagues on poly(itaconic acid)-based SAPs by inverse suspension polymerization found that the water absorbency of the prepared SAP particles depended on the washing solvent used to remove the surfactant [146]. Higher water content in the washing solvent resulted in greater absorbency, with 5 g/g for 100% ethanol and 14 g/g for a 50:50 mixture of ethanol and water. Less polar solvents caused the pores in the SAP particles to collapse (see Fig. 5 for SEM images), leading to lower absorbency values, while washing with water alone caused particle aggregation and difficulty in obtaining the product in powder form.
The SEM micrographs illustrating the effect of washing the particles with a pure ethanol and b 50:50 ethonal: water [149]. Reprinted from Fibers Polym., 22, Sand, A.; Shin, N. J.; Nam, H. G.; Kwark, Y. J Effects of Reaction Parameters on Water Absorption of Poly(Itaconic Acid) Superabsorbent Particles Synthesized by Inverse Suspension Polymerization, 898–903, Copyright (2021), with permission from Springer Nature.
Microwave-assisted polymerization
Microwave-assisted polymerization is another technique that generates free radicals during the polymerization process. Unlike suspension and solution radical graft copolymerization, where a chemical initiator is added, the free radicals in microwave-assisted polymerization are generated by thermal irradiation of the monomers and macromolecules [147]. However, Azad and colleagues reported microwave-assisted polymerization by adding small amounts of chemical initiator (ammonium persulfate) [148]. In another paper, Azad and colleagues reported a series of polymers synthesized by free-radical graft and microwave polymerization. The microwave synthesis resulted in more desirable properties such as shorter reaction time, higher absorption capacity, and centrifuge retention capacity [149,150,151]. A bio-composite SAP was synthesized using both microwave and conventional radical graft polymerization methods. The optimized reaction conditions indicated that the microwave approach achieved a high degree of grafting (%G), exhibited superior swelling properties, and reduced the polymerization reaction time compared to the optimized conventional method [150]. In the conventional method, the optimal time was determined to be 70 min, whereas the microwave method only took approximately 12 min. The maximum equilibrium swelling capacity achieved with microwave synthesis was around 1900 g/g, whereas the lowest was 1200 g/g. In another study, the use of a microwave method for surface cross-linking of poly(sodium acrylate)-based SAPs was investigated. The study used diglycidyl materials as surface cross-linking agents and N,N-Dimethylaniline as catalysts. Results showed that the microwave method significantly reduced the surface treatment time from 3 h to 4 min [151].
Non-radical polymerization
Free radical polymerization is a fast and easy method for producing superabsorbent polymers. However, controlling the formation of polymer network structure is challenging because the resulting network is often inhomogeneous; thus, the obtained polymer has decreased strength and absorption ability [98]. In non-radical polymerization, free radicals are not formed during polymer formation. The role of a chemical and thermal initiator is to create active sites which allow the grafting of the monomer onto the macromolecule. Non-radical polymerization involves grafting without the need for creating radical active sites. Instead, covalent bonds are formed between the reactive functional groups, such as carboxyl, hydroxyl, and amine, on the monomers and the polysaccharide macromolecules. The Michael addition polymerization is a non-radical polymerization process that is strongly influenced by reaction conditions such as temperature and monomer concentration (see Scheme 3) [152,153,154,155]. Sashiwa and colleagues reported a Michael addition reaction, where an acrylic acid monomer was grafted on a chitosan polysaccharide [152, 156, 157]. Porous polymers were synthesized using Michael reactions of multifunctional acrylate and bifunctional compounds in DMSO [155]. In this reaction, the reaction conditions were altered by varying the reaction temperature (20–50 °C), the concentration of the monomer (20–25 wt%), and the ratios of two macromolecules, trimethylolpropane propoxylate triacrylate (TPT) and hexamethylene diamine (HDA). The porous structure formation was controlled by the reaction conditions, with connected globules or co-continuous monolithic structures being formed. The TPT-HDA porous polymer absorbed various solvents, particularly CHCl3, and showed color variation based on reaction time and temperature. Thio-Michael reactions of TPT-HDT in DMSO with a photobase catalyst yielded porous polymers under specific conditions with controlled porous structures. It is reported that Michael addition reaction may lead to uncontrollable polymerization, so Fujita and colleagues conducted thermal and kinetic analyses experiments using an acrylic acid monomer to gain more understanding. The GPC analysis exposed that the products of Michael addition reaction were dimers, trimers, and tetramers, with a conversion rate of 82%. The kinetic analysis discovered that the Michael addition reaction had an order of 2.5, and the overall reaction rate constant was k = 3.52 × 103 × exp (− 1.18 × 105/T [K]) L1.5 mol−1.5 s−1 [158]. In another study, the impact of kinetic parameters on the overall reaction rates of base-catalyzed Michael reactions was evaluated using kinetic models [159]. The analysis was conducted on eight ternary thiol-Michael systems consisting of thiol–acrylate–vinyl sulfone and 1-thiol 2-vinyl. The model predicted the kinetic paths for the reaction and accompanying polymerization and individual parameters for propagation and chain transfer steps. Finally, the model was validated for network-forming polymerizations. The results provide guidance for selecting monomers to design thiol-Michael-based polymers with desired kinetic properties and material characteristics.

Adapted from Macromol. Biosci., 3, Sashiwa, H.; Yamamori, N.; Ichinose, Y.; Sunamoto, J.; Aiba, S., Chemical Modification of Chitosan, 17, 231–233 Copyright (2003), with permission from WILEY–VCH Verlag GMbH & Co. KGaA, Weinheim.
Non-radical polymerization of chitosan by the Michael addition reaction [156].
Cross-linking of SAPs can be achieved through physical or chemical methods, and Table 2 outlines the key distinctions between the two.
Table 3 lists the various advantages and disadvantages for the above mention polymerization methods.
A computational approach to polymer formation
All the SAP formation techniques mentioned above have their advantages, but they also have their drawbacks. In polymer chemistry, researchers mainly use a time- and resource-consuming trial-and-error approach to develop new polymer products. Many experimental conditions must be tested to discover the most optimal conditions for obtaining SAPs with desirable properties. Computational chemistry techniques such as central composite design (CCD) have an enormous potential in developing cross-linked polymer networks with desirable properties in shorter times and with minimal experimental resources. RSM (response surface methodology) is a statistical method for improving experimental efficiency and obtaining optimal results while minimizing trial-and-error laboratory experimentation, which can be costly [173,174,175,176]. CCD is the most extensively used RSM approach to date. Kown and colleagues used a computational technique (response surface methodology-central composite design (RSM-CCD)) to determine optimum conditions for cross-linking a bio-based SAP using itaconic acid as the monomer [175]. Their calculations considered the type of cross-linker, the amount and the reaction time. The conditions most effective for chemical cross-linking are: a reaction temperature of 160 °C, 2.22 mol % chemical cross-linker, and 8.7 min of reaction time.
Moreover, Yilmaz and colleagues report a superabsorbent polymer composed of poly(acrylic acid/kryptofix 23-dimethacrylate) was synthesized to treat a variety of toxic metals (Co, Ni, Cu, Cd, Mn, Zn, Pb, Cr, and Fe ions) in wastewater [177]. Computational tools were also utilized to gain additional insight into the SAP’s geometry and absorption factors, including ΔHf0 (heat of formation), d (bond length), φ (internal rotational angle), Estr (strain energy), ℓ (lengths of the repeating unit of the polymer chain), and ΔE (internal barrier energy) of stable conformations of the cross-linker and superabsorbent. Quantum chemical calculations were performed using Assisted Model Building with Energy Refinement (AMBER), molecular mechanic (MM2), and optimized potentials for liquid simulations (OPLS) for conformational analysis. The computational data indicated that the internal cavity radius of the polymer was 6.8928 Å, which could predict the absorption of metals and molecules by the polymer based on their size without the need for physical experimentation. The OPLS method’s lowest ΔE values also suggested that the SAP was more flexible in liquid reaction medium simulations. Figure 6 depicts the optimized structure of the polymer as determined by molecular mechanic (MM2) modeling.
The optimized structures of poly(acrylic acid/kryptofix 23-dimethacrylate) SAP as determined by the molecular mechanic computational method [177]. Reprinted from M. Materials (Basel)., 13, Savaskan Yilmaz, S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E., Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation, 1–23, Copyright (2020), with permission from MDPI Molecules.
Other computational tools, such as density functional theory (DFT) and computational molecular design (CMD), aid in the mechanistic understanding and structure optimization of macromolecules [178, 179]. The obtained information provides insight into the macromolecular physical and structural properties, which ultimately affect the materials' behavior when exposed to certain stimuli. Since most plastic applications require long-lasting materials, synthesizing durable bio-plastics has become a key commercial objective. Biomass chemicals with high hydrocarbon content, such as terpenes found in the resin of conifer trees, have gained significant attention as potential alternatives for developing new polymers [180]. The primary component of tree resin, α-pinene, consists of hydrocarbon molecules with stereo-complexity and unsaturated moieties that can undergo olefin polymerization. The δ-pinene monomer is made from α-pinene through a metal-free three-step synthesis process. Yarolimek’s research provides experimental and NMR data indicating that δ-pinene can be polymerized through ring-opening metathesis, a mechanism supported by DFT calculations revealing a ring strain energy of (∼ 35 kJ mol–1).
The utilization of mixtures containing SAPs and cement-based materials has become increasingly popular in commercial applications due to the SAPs’ ability to reduce water flow through the cracks found in cement-based products. As a result, Rodríguez and colleagues developed a mathematical lattice network model that examined water absorption in cement mortars in the presence and absence of SAPs [181]. The reduction of water flow in SAP-cement composites is believed to be due to the SAPs' capacity to absorb water and expand within the cracks, although there has been little research to confirm this hypothesis. Diffusion-controlled mode was utilized for the swelling kinetics of SAPs to simulate their water absorption during sorptivity testing in mortars. This model is a numerical model that describes the swelling kinetics of a SAP particle, suggesting that swelling is governed by a diffusion process and Esteves validated this by measuring the change in diameter of a SAP overtime [181, 182]. While the simulated and experimental data did not align perfectly, the general behavior of the curve was captured in the simulations, thus validating the law’s suitability for describing the water uptake of SAPs incorporated into a cementitious matrix.
Machine learning is a CMD technique fast gaining popularity in chemistry because of its potential to fast-track the discovery and design of innovative materials. Using machine learning-assisted methodology, Kondo and colleagues discovered a series of new polymers with the desired high thermal conductivity [183]. They used an algorithm to recognize quantitative properties relating to structure, thermal conductivity, and other targeted polymeric properties. From this, thousands of possible hypothetical polymers were generated, and based on ease of processing and synthetic accessibility, only three were selected for synthesis. The synthesized polymers achieved thermal conductivity between 0.18 and 0.41 W/mK, and these values are similar to some high-end commercial polymers. The ability to predict the performance of SAPs using computational or theoretical chemistry is a significant advantage for commercial manufacturers.
From the above, it is evident that it is worthwhile for polymer scientists to consider incorporating computational chemistry in their research as it is a promising predictive tool that can minimize trial-and-error experimentations and ultimately reduce operational costs [184, 185].
Factors affecting absorption/swelling
The swelling kinetics of a SAP refers to the quantity of liquid it can absorb over time, while its retention capacity represents the maximum amount of liquid that the hydrogel can chemically retain when surface water has been removed [133, 149]. Factors such as particle size, solution pH, ionic strength of saline solution, and degree of cross-linking may affect swelling capacity, kinetics, and retention [186, 187].
Ionic strength and pH stability
Examining the durability of SAPs across different pH levels and saline environments represents a significant aspect of the synthesis of SAPs. The difference in osmotic pressure inside and outside of the SAP is the driving force for solution absorption. Therefore, increasing the amount of cations (Na+) in the surrounding SAP solution by adding NaCl lowers the osmotic pressure and thus reduces the absorption capacity [160]. Thus maximum absorption occurs with deionized water and the lowest with a saline solution [73, 188, 189]. For the practical application of SAPs in hygiene products like diapers and sanitary pads, it is crucial to consider the ionic strength of the solutions for which they will be used. These solutions have higher ionic strength compared to deionized water, so it is essential to test SAPs under such conditions. The sensitivity of SAPs to the ionic strength of a solution is generally tested in a 0.9% NaCl solution which correlates to the salt concentration of urine. Peng and colleagues reported a starch-based SAP with maximum absorption of 1493.1 g/g in deionized water and a reduced absorption of 91.0 g/g in saline [190]. In another study, Zhao and colleagues introduced a Sulfamic Acid-Modified Starch with “salt-tolerant” properties, which exhibited better absorption of saline solutions than previously reported starch SAPs [191]. However, despite the improvement, the SAP’s absorption capacity was still lower in saline solution (145 g/g) compared to deionized water (1026 g/g). The term “salt-tolerant” is used to describe the SAPs because the sulfamic acid-modified SAP exhibits an improved water absorption capacity in the presence of salt solutions compared to starch-grafted acrylic acid SAPs. Figure 7 illustrates the reduction in absorption capability of several starch-based SAPs when exposed to saline solution relative to their performance in deionized water.
Graphical representation of absorption capacity of a series of acid-treated starch SAPs in deionized water versus 0.9% saline solution [191]. Reprinted from ACS Omega, 4, Zhao, C.; Zhang, M.; Liu, Z.; Guo, Y.; Zhang, Q Salt-Tolerant Superabsorbent Polymer with High Capacity of Water-Nutrient Retention Derived from Sulfamic Acid-Modified Starch, 5923–5930, Copyright (2019), with permission from American Chemical Society.
The decrease in absorption capacity in saline is not exclusive to starch- or bio-based polymers. Xu and colleagues reported a group of sodium polyacrylate–polyacrylamide SAPs that also decreased in absorption capacity in saline solutions. The SAPs had a maximum absorption of 1954 g/g in deionized water and 115 g/g in 0.9% saline solution [38]. From the above, it is evident that ions in solution hinder the absorption capacity of SAPs.
Moreover, Czarnecka and colleagues postulate that SAPs exhibit a charge screening effect, resulting in decreased absorption of solutions [73]. Consequently, salt solutions containing polyvalent/ multivalent cations reduce the absorption of the SAP to a greater extent than those with univalent cations. Ultimately, the presence of these ions triggers ionic cross-linking of the polymer, leading to a significant reduction in SAP absorption. Consequently, a rise in the concentration of salt ions in the solution will further reduce the absorption capacity. This is demonstrated by comparing the absorption of tap water to that of saline solution, which contains more concentrated free ions. Therefore, increasing the sodium concentration to the level found in blood (3.2 mg/mL, equivalent to ~ 0.32%) would result in an even greater decrease in the SAP’s absorption capacity. Hence, while developing SAPs for sanitary products, it is crucial to produce products that are resistant to salt, as the presence of ions in blood and urine can impact the absorption capacity, which can directly impact the consumer.
Studies show that the pH of the surrounding environment also affects the water absorption capacity of SAPs. Typically, the impact of pH on both the water absorption and structure of SAPs is evaluated by immersing the dehydrated SAPs in solutions with the same ionic strengths with different pH levels. Generally, maximum water absorption occurs at alkaline pH levels ranging between 6 and 10 [187, 189, 192, 193]. In their research, Adair and colleagues examined how fluids with pH values ranging from 4 to 10 affect the water absorption capacities of SAPs. Their findings show that the absorption capacity of SAPs is affected by pH values, with the highest absorption capacity observed at a pH solution of 8 (382 g/g). When the pH level of the surrounding medium is acidic, the polymer’s anionic carboxylate groups become protonated by the dissociated hydrogen atoms produced from the acid [186]. This induces hydrophobic effects on the polymer, resulting in a decrease in its swelling capacity.
Similarly, when the pH levels are basic, the swelling capacity of the polymer is decreased due to an excess of cations, which reduces the osmotic pressure required to transport liquid into the polymer [192]. The normal blood pH level is approximately 7.4, within the range of maximum absorption for most SAPs [194, 195]. For application in sanitary products, factors like lifestyle, disease, and food may alter a person’s blood and urine pH, thus affecting the absorption capacity negatively or positively depending on the individual’s overall pH.
Kinetics and particle size
During the swelling process, the water molecules permeate the polymer by diffusion and capillary forces and it has been reported that smaller particle sizes have a faster absorption rate for water molecules [187]. In adsorption, smaller particles tend to have a larger surface area, leading to faster and larger uptake of gases and solutions [196,197,198]. Fort and colleagues reported absorption values for three commercially available SAPs with varying average particle sizes; Creaorb (\(63\) µm), Cablock (291 µm), and Hydropam (526 µm) [187]. Cablock had the highest reported absorption value of around 250 g/g, and Hydropam had a value of 75 g/g. Initially, the fastest absorption was found for Creasorb due to its particle size, but it was retarded by the internal resistance of liquid transportation between the SAP particles. Additionally, if homogenization techniques are not employed, smaller particles tend to agglomerate, which may affect the kinetics. To investigate absorption kinetics, the tea bag method is the most popular technique [133, 160, 199, 200]. However, these methods have limitations, such as overestimating absorption capacity and gel leakage into the surrounding solution. Therefore, in recent years, other methods, such as centrifugal and vessel filtration cloth (VFC), have been introduced [187, 199].
Degree of cross-linking and monomers
The last step in SAP production involves attaching the monomer chain to the macromolecule with the help of a cross-linking agent to generate three-dimensional networks [73]. Thus the degree of cross-linking and amount of monomer play a role on the absorption properties of SAPs. Typically, SAPs with a high degree of cross-linking have high gel strength but low absorption capacity [160]. This is because a high degree of cross-linking leads to a more rigid and compact polymer, making it resistant to water uptake and as a result, less susceptible to swelling. Czarnecka and colleagues reported that increasing the concentration of the cross-linking agent reduces the swelling capacity of the polymer [73]. Additionally, cross-linking is responsible for the insolubility of SAPs in water and other aqueous solutions [46]. Regarding desorption, highly cross-linked SAPs exhibit a faster rate of liquid desorption compared to those with fewer cross-links, as reported by Yun and colleagues [160]. This is because highly cross-linked SAPs possess stronger retraction forces, which compel the absorbed liquid to exit the SAP more forcefully than less cross-linked SAPs.
Research has shown that monomers added to biodegradable polymers (such as polysaccharide), play a significant role on the swelling capacity of a SAP. Some of the most popular monomers used are acrylamide and acrylic acid because they contain hydrophilic functional groups (CONH2 and –COOH), which are essential for the swelling properties of SAPs [201]. When the monomer is neutralized, free ions that contribute to osmotic pressure are created, giving the polymer macromolecule a charge density. This facilitates water uptake, resulting in an increased swelling capacity of the SAP when the degree of neutralization is raised, assuming the level of cross-linking is fixed [136, 148, 149, 192]. However, there is a limit to this effect; after a certain degree of neutralization, the free ions on the polymer backbone will no longer increase, even if the degree of neutralization continues to rise. Therefore, a maximum water absorption level will be reached, regardless of any further neutralization of the monomer. The optimum degree of neutralization is reported to be approximately 70% (see Fig. 8a) [138, 149].
Graph illustrating the influence of (left) the degree of neutralization of the monomers and b the cellulose: monomer ratio on the swelling capacity of the SAP. Adapted from J. Polym., Guan, H.; Li, J.; Zhang, B.; Yu, X., Properties and Humidity Resistance Enhancement of Biodegradable Cellulose-Containing Superabsorbent Polymer, 1–8, Copyright (2017), with permission from Hindawi [138].
Furthermore, as with neutralization, there is a threshold beyond which an increase in added monomer content leads to a decrease in water absorption. Reports suggest that the optimal ratio of polysaccharide to monomer is 1:10, see Fig. 8b. Exceeding this ratio results in a reduction in the absorption of both water and saline solutions [134, 138, 202]. Continuously increasing the monomer beyond the absorption threshold increases the viscosity of the reaction mixture, hindering the movement of free radicals and monomers. Consequently, the probability of collisions between the monomers and macromolecules decreases, leading to homopolymerization instead of graft polymerization.
Moving toward antibacterial/fungal and self-cleaning sanitary products:
Vaginal flora and infections associated with it
Bacteria are classified as gram positive or gram negative; depending on the gram staining technique, they stain purple and pink, respectively, as shown in Fig. 9. Gram-positive bacteria possess a thick layer of peptidoglycan and lack an outer lipid membrane, while gram-negative bacteria have a thin peptidoglycan layer and have an outer lipid membrane, which acts as an additional barrier [203]. Gram-negative bacteria secrete exo- and endotoxins, whereas gram-positive secretions are solely exotoxins (Fig. 10).
Cartoon illustration of how light excites electron to the conduction band, which in turn creates reactive oxygen species, which can decompose carbon-based compounds and microorganisms [229]. Reprinted from J. Phys. Chem. Lett., 5, Banerjee, S.; Pillai, S. C.; Falaras, P.; O’Shea, K. E.; Byrne, J. A.; Dionysiou, D. D., New Insights into the Mechanism of Visible Light Photocatalysis, 2543–2554, Copyright (2014), with permission from American Chemical Society.
Vaginal flora consists of good and bad bacteria. External stimuli like pH may cause an imbalance, resulting in vaginal infections. The vagina contains a healthy balance of bacteria and fungi, with lactobacillus being the “good bacteria” that prevents the overgrowth and invasion of other microorganisms by secreting hydrogen peroxide, lactic acid, and bacteriocin-liked substances [204,205,206,207]. Common symptoms of vaginal infections include itching, burning during urination, and an unpleasant odor. To minimize the risk of infection, it is clinically recommended that individuals frequently change their sanitary pads, tampons, and pantyliners. However, in underprivileged communities, women and girls cannot afford sanitary products, let alone change them frequently. As a result, this can have a detrimental impact on their lives and may occasionally be fatal. Some common infectious conditions caused by sanitary products include menstrual toxic shock syndrome (mTSS), bacterial vaginosis, and yeast infections.
The prolonged use of sanitary pads, pantyliners, and tampons creates a warm, sweaty environment, which are ideal conditions for the overgrowth of bacteria and fungi. This is defined as vaginal microbiota dysbiosis [205]. Vaginal yeast infections (candidal vulvovaginitis) and bacterial vaginosis occur when there is an overgrowth of fungus or bacteria, causing an imbalance in the vaginal flora. Gardnerella vaginalis is a gram-variable bacteria associated with bacterial vaginosis, and Candida albicans is the fungus associated with vaginal yeast infections [208, 209]. Although not fatal, vaginal candidiasis and bacterial vaginosis symptoms make daily life uncomfortable [210]. Another disease that may occur due to the prolonged use of certain menstrual products is menstrual toxic shock syndrome (mTSS). It is a rare but lethal bacterial disease characterized by rash, fever, skin shedding, and hypotension, leading to organ failure [211,212,213]. Exotoxins produced by gram-positive bacteria cause the infection, Staphylococcus aureus and group A streptococci [211, 212]. Other groups of streptococci, particularly B, C, and G, are now suspected of causing rare streptococcal mTSS. This fatal disease is associated with the prolonged (> 6 h) insertion of intravaginal sanitary devices such as super absorbing tampons, menstrual cups, and UID, among others [214]. The disease is often misdiagnosed, and treatment is delayed, resulting in fatalities or patient hospitalization [215].
Natural-based materials as a strategy for inducing antibacterial properties
From the above, it is clear that synthetic personal hygiene products may have detrimental effects on the end user. Hence, developing biocompatible sanitary products with antibacterial and self-cleaning properties should be a social and health priority, especially in low-income households where women cannot afford essential sanitary products and healthcare when they develop infections. The personal hygiene industry is highly dependent on the production of SAPs, and the most widely used SAP is petroleum-based sodium polyacrylate. In light of new research showing the detrimental effects of petroleum-based SAPs on the environment and human health, SAP research is moving toward a greener and more sustainable route. Some feminine hygiene products (tampons, sanitary pads, and pantyliners) are marketed as 100% biodegradable and environmentally friendly. Most are comprised entirely of organic fibers and do not contain SAPs. Thus, their liquid absorption and retention capacity are low [216]. Those with SAPs containing petroleum-based sodium polyacrylate incorporated within the organic fibers are not 100% environmentally friendly or biocompatible and may contain toxic chemicals.
Sun and colleagues reported the effects of using different types of pads on the inflammation and the flora of the vagina [217]. The study showed that using bio-based sanitary pads like hemp can reduce a woman’s proneness to have reproductive tract infections by maintaining the balance of the vaginal flora. Some polysaccharides, such as chitin and chitosan, have natural antibacterial properties. Benhabiles and colleagues have reported that chitin is active against gram-positive bacteria, including Staphylococcus aureus and group A streptococci, associated with the fatal mTSS [218]. Chitosan is active against both gram-positive and gram-negative bacteria. It also has antifungal properties against Candida albicans; therefore, when it is incorporated into personal hygiene products, it has the potential to act against both bacteria and fungi [219]. Chitosan also has exceptional mucoadhesive properties and a safety profile, making it a promising candidate for enhancing localized vaginal therapy. Jøraholmen and colleagues conducted a study investigating the efficacy of chitosan-coated liposomes and chitosan hydrogel as drug carriers in the vaginal tract [220]. The study confirmed the antibacterial activity of these formulations against Staphylococcus epidermidis and Staphylococcus aureus. Chitosan offers an added benefit of closely interacting with mucus, facilitating an effective contact time between the formulation and the epithelial lining of the vaginal mucus [221]. This mucoadhesive property of chitosan also gives it the potential to be used in menstrual cups. Bfree Cup is an antibacterial menstrual cup made up of 100% medical-grade silicone; hence, it has no synthetic chemicals or toxins [222]. Unlike conventional cups, the Bfree antibacterial cup does not need to be boiled between uses, its antibacterial property come from the physically created superhydrophobic surface of the cup, which is claimed to prevent the formation of a biofilm. It only requires rinsing and wiping. A similar alternative to the BCup is the ElleCup which is also reusable and is made up of 100% medical-grade silicone, this is not antibacterial but comes with antibacterial spray.
Nanotechnology as a strategy for inducing antibacterial and self-cleaning properties
Nanotechnology holds significant commercial prospects in the context of making reusable sanitary products. It introduces a new idea of self-cleaning textiles, providing clean and fresh daily clothes. The antibacterial and self-cleaning properties of these reusable sanitary products can be induced by fixating nanoparticles onto fabrics. Two primary methods exist for inducing self-cleaning properties through nanotechnology, namely (1) dirt- and water-repellent materials as well as (2) photocatalytic degradation of organic matter [223].
With the first method, fabric fibers are given a protective coating by bonding nanoparticles to them and then attaching hydrophobic functionalities that can directly repel water, dirt, and bacteria to the nanoparticles. This results in self-cleaning and antibacterial properties. The technique of inducing antibacterial properties on fabric involves fixating a metal oxide or metal nanoparticle photocatalyst onto the fabric [224,225,226,227]. This area of science can be used on the fabric of reusable sanitary pads to minimize possible infections. Generally, fixation is achieved by sol–gel or pad dry cure technique with a binder to ensure that the catalyst adheres to the fabric [223, 228]. When the coated fabric is exposed to light, photons with higher energy than the band gap of the photocatalyst transfer electrons to the conduction band. The excited electrons react with the oxygen atoms in the air, generating oxygen radicals. Unstable oxygen radicals react to decompose carbon-based compounds through oxidation–reduction reactions, breaking down organic substances like dirt and microorganisms into safe substances such as carbon dioxide and water (see Fig. 11 for the photocatalytic process). The metal oxide is only a catalyst and is not consumed during the reaction, enabling the coating to remove stains continuously as shown in Fig. 11.
Photograph showing the self-cleaning effect of fabric treated with TiO2 nanoparticles stained with coffee after expose to sun light [223]. Reprinted from J. Text. Inst., Pillai, O. M.; Sundaramoorthy, S., Development of Photocatalytic Self-Cleaning Textiles Using Tin Oxide and Titanium Dioxide Nanoparticles Mixture, 674–681, Copyright (2022), with permission from Taylor and Francis.
Photocatalytic degradation of dye and pharmaceutical waste has been studied extensively in water purification. However, the application of this technique for cleaning textiles is still minimal. In a study conducted by Kavitha–Sankar, using antibacterial composites of cellulose pulp and silver nanoparticles (< 100 nm) in sanitary napkins were tested for biological safety. The testing included in vivo vaginal irritation, in vitro cytotoxicity, and intracutaneous reactivity [230]. The cytotoxicity results showed that the silver nanoparticle incorporated cellulose is non-cytotoxic, and no skin irritation was observed in the animal test subjects. These results indicate that the silver-cellulose composite has good biocompatibility, making it a safe hygiene product that meets the recommended standards. Another study used chitosan from beetle exoskeletons to synthesize MgO and ZnO nanoparticles [231]. The ZnO nanoparticles showed stronger antibacterial activity against gram-positive bacteria than gram-negative bacteria, while MgO nanoparticles showed the opposite trend. The findings show a synergystic effect between the chitosan polysaccharide and metal nanoparticles as antibacterial agents. Maghsoudi and colleagues aimed to develop a green synthesis method using Achillea millefolium plant extract to create ZnO NPs, Ag NPs, and Ag/ZnO nanocomposite with antibacterial properties [232]. Antibacterial activity was tested against Pseudomonas aeruginosa, Escherichia coli, Bacillus cereus, and Staphylococcus aureus, with the Ag/ZnO nanocomposite showing the highest activity against E. coli. The nanocomposite also displayed better antibacterial activity than pure Ag and ZnO nanoparticles. Additionally, Ag/ZnO nanocomposite-loaded cotton fiber showed strong antibacterial activity, even after multiple washes. This demonstrates how nanotechnology can add antibacterial properties to reusable sanitary products, thereby enhancing the quality of life for young women who may not have the means to afford disposable ones.
Conclusion
This article provides insights into the developments and issues of SAPs for managing menstrual health, emphasizing the need for safer and more long-lasting solutions while addressing environmental issues. Exploring various SAP types, their preparation, absorption, and swelling factors, as well as techniques for antibacterial and self-cleaning qualities, provides insightful information. Additionally, incorporating nanotechnology into sanitary items has the potential to improve their use and hygienic standards. Although they currently have higher costs and worse performance when compared to synthetic alternatives, the growing interest in bio-modified or natural-based SAPs is in line with the global commitment to environmental conservation. The development of natural-based SAPs becomes increasingly required because to the high volume of SAP consumption in sanitary applications and the rising price of crude oil, opening the path the way for future advancements in this field.
Data availability
Not applicable (N/A).
References
Hennegan J, Dolan C, Wu M et al (2016) Measuring the prevalence and impact of poor menstrual hygiene management: a quantitative survey of schoolgirls in rural Uganda. BMJ Open 6:e012596. https://doi.org/10.1136/bmjopen-2016-012596
Sommer M, Hirsch JS, Nathanson C, Parker RG (2015) Comfortably, safely, and without shame: defining menstrual hygiene management as a public health issue. Am J Public Health 105:1302–1311. https://doi.org/10.2105/AJPH.2014.302525
Rossouw L, Ross H (2021) Understanding period poverty: socio-economic inequalities in menstrual hygiene management in eight low- and middle-income countries. Int J Environ Res Public Health 18:2571. https://doi.org/10.3390/ijerph18052571
Das P, Baker KK, Dutta A et al (2015) Menstrual hygiene practices, WASH access and the risk of urogenital infection in women from Odisha, India. PLoS ONE 10:e0130777. https://doi.org/10.1371/journal.pone.0130777
Kuhlmann AS, Henry K, Wall LL (2017) Menstrual hygiene management in resource-poor countries. Obstet Gynecol Surv 72:356–376. https://doi.org/10.1097/OGX.0000000000000443
Hennegan J, Shannon AK, Rubli J et al (2019) Women’s and girls’ experiences of menstruation in low- and middle-income countries: a systematic review and qualitative metasynthesis. PLoS Med 16:e1002803. https://doi.org/10.1371/journal.pmed.1002803
Hennegan J, Montgomery P (2016) Do menstrual hygiene management interventions improve education and psychosocial outcomes for women and girls in low and middle income countries? A systematic review. PLoS ONE 11:e0146985. https://doi.org/10.1371/journal.pone.0146985
Mehta S, Grover A, Mittal N et al (2022) Reusable sanitary napkins—time to revisit. J Public Health (Bangkok) 44:356–362. https://doi.org/10.1093/pubmed/fdaa192
Sathishkumar G, Aarthi M, Senthilkumar R et al (2019) Biodegradable cellulosic sanitary napkins from waste cotton and natural extract based anti-bacterial nanocolorants. J Indian Inst Sci 99:519–528. https://doi.org/10.1007/s41745-019-00123-x
Sivakami M, Maria van Eijk A, Thakur H et al (2019) Effect of menstruation on girls and their schooling, and facilitators of menstrual hygiene management in schools: surveys in government schools in three states in India, 2015. J Glob Health 9:010408. https://doi.org/10.7189/jogh.09.010408
Siegel L (2017) The eco guide to sanitary products. In: Guard, p 1. https://www.theguardian.com/environment/2017/oct/29/the-eco-guide-to-period-dramas.
Park CJ, Barakat R, Ulanov A et al (2019) Sanitary pads and diapers contain higher phthalate contents than those in common commercial plastic products. Reprod Toxicol 84:114–121. https://doi.org/10.1016/j.reprotox.2019.01.005
Chen J, Wu J, Raffa P et al (2022) Superabsorbent polymers: from long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog Polym Sci 125:101475. https://doi.org/10.1016/j.progpolymsci.2021.101475
Bashari A, Rouhani Shirvan A, Shakeri M (2018) Cellulose-based hydrogels for personal care products. Polym Adv Technol 29:2853–2867. https://doi.org/10.1002/pat.4290
Su Y, Wenzel M, Paasch S et al (2022) One-pot synthesis of brewer’s spent grain-supported superabsorbent polymer for highly efficient uranium adsorption from wastewater. Environ Res 212:113333. https://doi.org/10.1016/j.envres.2022.113333
Sujitha VS, Ramesh B, Xavier JR (2023) Effect of superabsorbent polymer hydrogels in the advancement of cementitious materials—a review. J Polym Environ 31:2761–2778. https://doi.org/10.1007/s10924-023-02782-5
Oladosu Y, Rafii MY, Arolu F et al (2022) Superabsorbent polymer hydrogels for sustainable agriculture: a review. Horticulturae 8:605. https://doi.org/10.3390/horticulturae8070605
Sharma S, Anwar MF, Dinda AK et al (2022) Polyaspartic acid, 2-acrylamido-2-Methyl propane sulfonic acid and sodium alginate based biocompatible stimuli responsive polymer gel for controlled release of GHK-Cu peptide for wound healing. J Biomater Appl 37:132–150. https://doi.org/10.1177/08853282221076708
Sonkaya E, Kürklü ZGB, Bakır Ş (2021) Effect of polymerization time on residual monomer release in dental composite. in vitro study. Int J Polym Sci 2021:1–8. https://doi.org/10.1155/2021/8101075
Milani P, França D, Balieiro AG, Faez R (2017) Polymers and its applications in agriculture. Polímeros 27:256–266. https://doi.org/10.1590/0104-1428.09316
Chang L, Xu L, Liu Y, Qiu D (2021) Superabsorbent polymers used for agricultural water retention. Polym Test 94:107021. https://doi.org/10.1016/j.polymertesting.2020.107021
Ashraf AM, Ragavan T, Begam SN (2021) Superabsorbent polymers (SAPs) hydrogel: water saving technology for increasing agriculture productivity in drought prone areas: a review. Agric Rev 42:183–189. https://doi.org/10.18805/ag.R-2023
Takahashi K, Muraoka R, Otsuka K (2020) Technology adoption, impact, and extension in developing countries’ agriculture: a review of the recent literature. Agric Econ 51:31–45. https://doi.org/10.1111/agec.12539
Ostrand MS, DeSutter TM, Daigh ALM et al (2020) Superabsorbent polymer characteristics, properties, and applications. Agrosyst Geosci Environ 3:e20074. https://doi.org/10.1002/agg2.20074
He Z, Shen A, Guo Y et al (2019) Cement-based materials modified with superabsorbent polymers: a review. Constr Build Mater 225:569–590. https://doi.org/10.1016/j.conbuildmat.2019.07.139
Mechtcherine V (2016) Use of superabsorbent polymers (SAP) as concrete additive. RILEM Tech Lett 1:81. https://doi.org/10.21809/rilemtechlett.2016.18
Zhang Y, Chen Y, Alfred M et al (2021) Alkaline sodium polyacrylate-starch hydrogels with tolerance to cold conditions for stretchable zinc-air batteries. Compos Part B Eng 224:109228. https://doi.org/10.1016/j.compositesb.2021.109228
Mo F, Guo B, Ling W et al (2022) Recent progress and challenges of flexible Zn-based batteries with polymer electrolyte. Batteries 8:59. https://doi.org/10.3390/batteries8060059
Bachra Y, Grouli A, Damiri F et al (2020) A new approach for assessing the absorption of disposable baby diapers and superabsorbent polymers: a comparative study. Results Mater 8:100156. https://doi.org/10.1016/j.rinma.2020.100156
Yang L, Liu H, Ding S et al (2020) Superabsorbent fibers for comfortable disposable medical protective clothing. Adv Fiber Mater 2:140–149. https://doi.org/10.1007/s42765-020-00044-w
Vasile C, Pamfil D, Stoleru E, Baican M (2020) New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 25:1539. https://doi.org/10.3390/molecules25071539
Leigh T, Fernandez-Trillo P (2020) Helical polymers for biological and medical applications. Nat Rev Chem 4:291–310. https://doi.org/10.1038/s41570-020-0180-5
Elliot M (2013) Superabsorbent polymers. BASF Aktiengesellschaft, Ludwigshafen
Gontia P, Janssen M (2016) Life cycle assessment of bio-based sodium polyacrylate production from pulp mill side streams: case study of thermo-mechanical and sulfite pulp mills. J Clean Prod 131:475–484. https://doi.org/10.1016/j.jclepro.2016.04.155
Chu HS, Ahn J-H, Yun J et al (2015) Direct fermentation route for the production of acrylic acid. Metab Eng 32:23–29. https://doi.org/10.1016/j.ymben.2015.08.005
Pramod CV, Fauziah R, Seshan K, Lange J-P (2018) Bio-based acrylic acid from sugar via propylene glycol and allyl alcohol. Catal Sci Technol 8:289–296. https://doi.org/10.1039/C7CY01416C
Dishisha T, Pyo S-H, Hatti-Kaul R (2015) Bio-based 3-hydroxypropionic- and acrylic acid production from biodiesel glycerol via integrated microbial and chemical catalysis. Microb Cell Fact 14:200. https://doi.org/10.1186/s12934-015-0388-0
Xu T, Zhu W, Sun J (2022) Structural modifications of sodium polyacrylate-polyacrylamide to enhance its water absorption rate. Coatings 12:1234. https://doi.org/10.3390/coatings12091234
Braun O, Coquery C, Kieffer J et al (2021) Spotlight on the life cycle of acrylamide-based polymers supporting reductions in environmental footprint: review and recent advances. Molecules 27:42. https://doi.org/10.3390/molecules27010042
Osman I, Seyfullah K, Burcu C (2005) The effect of PEG on the water absorption capacity and rate of superabsorbent copolymers based on acrylic acid. Int J Polym Mater 54:1001–1008. https://doi.org/10.1080/009140390517704
Tokiwa Y, Calabia B, Ugwu C, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10:3722–3742. https://doi.org/10.3390/ijms10093722
Polyacrylamide. In: Campaign Safe Cosmet. https://www.safecosmetics.org/chemicals/polyacrylamide/. Accessed 18 May 2023
Panico A, Serio F, Bagordo F, Grassi T, Idolo A, De Giorgi M, Guido M, Congedo M, De Donno A (2019) Skin safety and health prevention: an overview of chemicals in cosmetic products. J Prev Med Hyg 60:50–57. https://doi.org/10.15167/2421-4248/jpmh2019.60.1.1080
Azizi A, Kabiri K, Zohuriaan-Mehr MJ et al (2018) Transamidation: a feasible approach of surface modification to improve absorbency under load of agricultural superabsorbent materials. J Mater Res 33:2327–2335. https://doi.org/10.1557/jmr.2018.240
Chang S, Kim M, Oh S et al (2018) Multi-scale characterization of surface-crosslinked superabsorbent polymer hydrogel spheres. Polymer (Guildf) 145:174–183. https://doi.org/10.1016/j.polymer.2018.04.073
Kwon Y-R, Kim J-S, Kim D-H (2021) Effective enhancement of water absorbency of itaconic acid based-superabsorbent polymer via tunable surface—crosslinking. Polymers (Basel) 13:2782. https://doi.org/10.3390/polym13162782
Omidian H, Rocca JG, Park K (2005) Advances in superporous hydrogels. J Control Release 102:3–12. https://doi.org/10.1016/j.jconrel.2004.09.028
Gotoh T, Nakatani Y, Sakohara S (1998) Novel synthesis of thermosensitive porous hydrogels. J Appl Polym Sci 69:895–906. https://doi.org/10.1002/(SICI)1097-4628(19980801)69:5%3c895::AID-APP8%3e3.0.CO;2-H
Benda D, Šňupárek J, Čermák V (1997) Inverse suspension polymerization of the hydrophilic acrylic monomers in the static continuous phase. J Dispers Sci Technol 18:115–121. https://doi.org/10.1080/01932699708943723
Ajmal M, Anwar S, Naeem H et al (2020) Poly(acrylic acid) hydrogel microparticles fabricated with silver nanoparticles: synthesis, characterization, and catalytic applications. Polym Eng Sci 60:2918–2929. https://doi.org/10.1002/pen.25523
Chen H, Huang H (2016) Fast-swelling superabsorbent polymers with polymerizable macromolecular surfactant as crosslinker. J Appl Polym Sci 133:44173. https://doi.org/10.1002/app.44173
Seera SDK, Kundu D, Banerjee T (2020) Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 27:6521–6535. https://doi.org/10.1007/s10570-020-03249-9
Gotovtsev PM, Badranova GU, Zubavichus YV et al (2019) Electroconductive PEDOT:PSS-based hydrogel prepared by freezing-thawing method. Heliyon 5:e02498. https://doi.org/10.1016/j.heliyon.2019.e02498
Adelnia H, Ensandoost R, Shebbrin Moonshi S et al (2022) Freeze/thawed polyvinyl alcohol hydrogels: present, past and future. Eur Polym J 164:110974. https://doi.org/10.1016/j.eurpolymj.2021.110974
Kabiri K, Omidian H, Zohuriaan-Mehr M (2003) Novel approach to highly porous superabsorbent hydrogels: synergistic effect of porogens on porosity and swelling rate. Polym Int 52:1158–1164. https://doi.org/10.1002/pi.1218
Boyandin AN, Dvoinina LM, Sukovatyi AG, Sukhanova AA (2020) Production of porous films based on biodegradable polyesters by the casting solution technique using a co-soluble porogen (camphor). Polymers (Basel) 12:1950. https://doi.org/10.3390/polym12091950
Lakshmi DS, Saxena M, Dass LA (2021) Maiden exploration of ulvan, a sulfated green seaweed polysaccharide as morphology-controlled porogen. Curr Res Green Sustain Chem 4:100096. https://doi.org/10.1016/j.crgsc.2021.100096
Czarnecka E, Nowaczyk J (2021) Synthesis and characterization superabsorbent polymers made of starch, acrylic acid, acrylamide, poly(vinyl alcohol), 2-hydroxyethyl methacrylate, 2-acrylamido-2-methylpropane sulfonic acid. Int J Mol Sci 22:4325. https://doi.org/10.3390/ijms22094325
Chen Z, Dong F, Liu M, Qi X (2011) Preparation and properties of porous poly(sodium acrylate- co -acrylamide) salt-resistant superabsorbent composite. Polym Eng Sci 51:2453–2464. https://doi.org/10.1002/pen.22034
Bao J, Chen S, Wu B et al (2015) A novel foaming approach to prepare porous superabsorbent poly(sodium acrylic acid) resins. J Appl Polym Sci 132:41298. https://doi.org/10.1002/app.41298
Mendoza L, Hossain L, Downey E et al (2019) Carboxylated nanocellulose foams as superabsorbents. J Colloid Interface Sci 538:433–439. https://doi.org/10.1016/j.jcis.2018.11.112
Cao L, Yang H, Zhou Y et al (2013) A new process for preparation of porous polyacrylamide resins and their humidity control properties. Energy Build 62:590–596. https://doi.org/10.1016/j.enbuild.2013.03.041
Yang M, Wu J, Graham GM et al (2021) Hotspots, frontiers, and emerging trends of superabsorbent polymer research: a comprehensive review. Front Chem. https://doi.org/10.3389/fchem.2021.688127
Pytlik E, Molino D, Moritz J (1996) Superabsorbent polymers. In: AMCOL NONWOVENS Mark. http://wwwcourses.sens.buffalo.edu/ce435/Diapers/Diapers.html
Wang Z, Ning A, Xie P et al (2017) Synthesis and swelling behaviors of carboxymethyl cellulose-based superabsorbent resin hybridized with graphene oxide. Carbohydr Polym 157:48–56. https://doi.org/10.1016/j.carbpol.2016.09.070
Gao J, Liu J, Peng H et al (2018) Preparation of a low-cost and eco-friendly superabsorbent composite based on wheat bran and laterite for potential application in Chinese herbal medicine growth. R Soc Open Sci 5:180007. https://doi.org/10.1098/rsos.180007
Somers MJ, Alfaro JF, Lewis GM (2021) Feasibility of superabsorbent polymer recycling and reuse in disposable absorbent hygiene products. J Clean Prod 313:127686. https://doi.org/10.1016/j.jclepro.2021.127686
Hosseini Ravandi SA, Valizadeh M (2011) Properties of fibers and fabrics that contribute to human comfort. In: Improving comfort in clothing. Elsevier, pp 61–78
Ramadan AR (2008) Characterization of biobleaching of Cotton/Linen Fabrics. In: J. Text. Appar. Technol. Manag. https://ojs.cnr.ncsu.edu/index.php/JTATM/article/view/311/251. Accessed 27 Feb 2023
(2022) Bio-based superabsorbents on the sanitary products market. In: World Bio Mark. https://worldbiomarketinsights.com/bio-based-superabsorbents-on-the-sanitary-products-market/. Accessed 9 Apr 2023
Aaliya B, Sunooj KV, Lackner M (2021) Biopolymer composites: a review. Int J Biobased Plast 3:40–84. https://doi.org/10.1080/24759651.2021.1881214
Alam MN, Islam MS, Christopher LP (2019) Sustainable production of cellulose-based hydrogels with superb absorbing potential in physiological saline. ACS Omega 4:9419–9426. https://doi.org/10.1021/acsomega.9b00651
Czarnecka E, Nowaczyk J (2020) Semi-natural superabsorbents based on starch-g-poly(acrylic acid): modification, synthesis and application. Polymers (Basel) 12:1794. https://doi.org/10.3390/polym12081794
Arredondo R, Yuan Z, Sosa D et al (2023) Performance of a novel, eco-friendly, cellulose-based superabsorbent polymer ( <scp>Cellulo-SAP</scp> ): absorbency, stability, reusability, and biodegradability. Can J Chem Eng 101:1762–1771. https://doi.org/10.1002/cjce.24601
Choi H, Park J, Lee J (2022) Sustainable bio-based superabsorbent polymer: poly(itaconic acid) with superior swelling properties. ACS Appl Polym Mater 4:4098–4108. https://doi.org/10.1021/acsapm.2c00021
Draney K, Bates J (2022) Biodegradable superabsorbent polymers. pp 727–735
Abdulhameed A (2022) Preparation of low-cost and eco-friendly superabsorbent based from rice husk cellulose cross-linked with boric acid using microwave. MOJ Ecol Environ Sci 7:121–126. https://doi.org/10.15406/mojes.2022.07.00257
Nnadi F, Brave C (2011) Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J Soil Sci Environ Manag 2:206–211
Capezza AJ, Lundman M, Olsson RT et al (2020) Carboxylated wheat gluten proteins: a green solution for production of sustainable superabsorbent materials. Biomacromol 21:1709–1719. https://doi.org/10.1021/acs.biomac.9b01646
Llanes L, Dubessay P, Pierre G et al (2020) Biosourced polysaccharide-based superabsorbents. Polysaccharides 1:51–79. https://doi.org/10.3390/polysaccharides1010005
Guan Y, Bian J, Peng F et al (2014) High strength of hemicelluloses based hydrogels by freeze/thaw technique. Carbohydr Polym 101:272–280. https://doi.org/10.1016/j.carbpol.2013.08.085
Lotfipour F, Alami-Milani M, Salatin S et al (2019) Freeze-thaw-induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: the experimental design and optimization. Res Pharm Sci 14:175. https://doi.org/10.4103/1735-5362.253365
Guo Y, Wu M, Li R et al (2022) Thermostable physically crosslinked cryogel from carboxymethylated konjac glucomannan fabricated by freeze-thawing. Food Hydrocoll 122:107103. https://doi.org/10.1016/j.foodhyd.2021.107103
Kim J, Choi J, Hyun J (2022) Free-form three-dimensional nanocellulose structure reinforced with poly(vinyl alcohol) using freeze-thaw process. Carbohydr Polym 298:120055. https://doi.org/10.1016/j.carbpol.2022.120055
Zhang H, Zhang F, Wu J (2013) Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React Funct Polym 73:923–928. https://doi.org/10.1016/j.reactfunctpolym.2012.12.014
García-Guzmán L, Cabrera-Barjas G, Soria-Hernández CG et al (2022) Progress in starch-based materials for food packaging applications. Polysaccharides 3:136–177. https://doi.org/10.3390/polysaccharides3010007
Chiu C, Solarek D (2009) Modification of starches. In: Starch. Elsevier, pp 629–655. https://doi.org/10.1016/B978-0-12-746275-2.00017-3
Mohamad N, Mohd Amin MCI, Pandey M et al (2014) Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: accelerated burn wound healing in an animal model. Carbohydr Polym 114:312–320. https://doi.org/10.1016/j.carbpol.2014.08.025
Faltermeier A, Behr M, Reicheneder C et al (2011) Electron-beam irradiation of polymer bracket materials. Am J Orthod Dentofac Orthop 139:e7–e11. https://doi.org/10.1016/j.ajodo.2009.10.038
Fekete T, Borsa J, Takács E, Wojnárovits L (2017) Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem Cent J 11:46. https://doi.org/10.1186/s13065-017-0273-5
Li R, Wu G (2020) Preparation of polysaccharide-based hydrogels via radiation technique. In: Hydrogels based on natural polymers. Elsevier, pp 119–148
Chen P, Zhang W, Luo W, Fang Y (2004) Synthesis of superabsorbent polymers by irradiation and their applications in agriculture. J Appl Polym Sci 93:1748–1755. https://doi.org/10.1002/app.20612
Nizam El-Din HM, Abd Alla SG, El-Naggar AWM (2010) Swelling and drug release properties of acrylamide/carboxymethyl cellulose networks formed by gamma irradiation. Radiat Phys Chem 79:725–730. https://doi.org/10.1016/j.radphyschem.2010.01.011
Ghobashy MM, Abd El-Wahab H, Ismail MA et al (2020) Characterization of Starch-based three components of gamma-ray cross-linked hydrogels to be used as a soil conditioner. Mater Sci Eng B 260:114645. https://doi.org/10.1016/j.mseb.2020.114645
Dispat N, Poompradub S, Kiatkamjornwong S (2020) Synthesis of ZnO/SiO2-modified starch-graft-polyacrylate superabsorbent polymer for agricultural application. Carbohydr Polym 249:116862. https://doi.org/10.1016/j.carbpol.2020.116862
Fekete T, Borsa J, Takács E, Wojnárovits L (2016) Synthesis of cellulose-based superabsorbent hydrogels by high-energy irradiation in the presence of crosslinking agent. Radiat Phys Chem 118:114–119. https://doi.org/10.1016/j.radphyschem.2015.02.023
Pourjavadi A, Zohuriaan-Mehr MJ, Ghasempoori SN, Hossienzadeh H (2007) Modified CMC. V. Synthesis and super-swelling behavior of hydrolyzed CMC-g-PAN hydrogel. J Appl Polym Sci 103:877–883. https://doi.org/10.1002/app.25224
Cheng B, Pei B, Wang Z, Hu Q (2017) Advances in chitosan-based superabsorbent hydrogels. RSC Adv 7:42036–42046. https://doi.org/10.1039/C7RA07104C
Haneef M, Ceseracciu L, Canale C et al (2017) Advanced materials from fungal mycelium: fabrication and tuning of physical properties. Sci Rep 7:41292. https://doi.org/10.1038/srep41292
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13:1133–1174. https://doi.org/10.3390/md13031133
TerkulaIber B, AzmanKasan N, Torsabo D, WeseOmuwa J (2022) A review of various sources of chitin and chitosan in nature. J Renew Mater 10:1097–1123. https://doi.org/10.32604/jrm.2022.018142
Cheng J, Zhu H, Huang J et al (2020) The physicochemical properties of chitosan prepared by microwave heating. Food Sci Nutr 8:1987–1994. https://doi.org/10.1002/fsn3.1486
Sivashankari PR, Prabaharan M (2017) Deacetylation modification techniques of chitin and chitosan. In: Chitosan based biomaterials Volume 1. Elsevier, pp 117–133. https://doi.org/10.1016/B978-0-08-100230-8.00005-4
Yilmaz Atay H (2019) Antibacterial activity of chitosan-based systems. In: Functional chitosan. Springer, Singapore, pp 457–489. https://doi.org/10.1007/978-981-15-0263-7_15
Fang S, Wang G, Xing R et al (2019) Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int J Biol Macromol 132:575–584. https://doi.org/10.1016/j.ijbiomac.2019.03.176
Mistry PA, Konar MN, Latha S et al (2023) Chitosan superabsorbent biopolymers in sanitary and hygiene applications. Int J Polym Sci 2023:1–14. https://doi.org/10.1155/2023/4717905
Li J, Tian X, Hua T et al (2021) Chitosan natural polymer material for improving antibacterial properties of textiles. ACS Appl Bio Mater 4:4014–4038. https://doi.org/10.1021/acsabm.1c00078
Li L, Thangamathesvaran PM, Yue CY et al (2001) Gel network structure of methylcellulose in water. Langmuir 17:8062–8068. https://doi.org/10.1021/la010917r
Aoyagi S, Onishi H, Machida Y (2007) Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm 330:138–145. https://doi.org/10.1016/j.ijpharm.2006.09.016
Mi F-L, Shyu S-S, Wu Y-B et al (2001) Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 22:165–173. https://doi.org/10.1016/S0142-9612(00)00167-8
Chenite A, Chaput C, Wang D et al (2000) Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 21:2155–2161. https://doi.org/10.1016/S0142-9612(00)00116-2
Richardson SM, Hughes N, Hunt JA et al (2008) Human mesenchymal stem cell differentiation to NP-like cells in chitosan–glycerophosphate hydrogels. Biomaterials 29:85–93. https://doi.org/10.1016/j.biomaterials.2007.09.018
Park JP, Koh M-Y, Sung PS et al (2015) Inactivation efficiency of DNA and RNA viruses during chitin-to-chitosan conversion. Macromol Res 23:505–508. https://doi.org/10.1007/s13233-015-3081-6
Thakur VK, Kessler MR (2016) Fire resistance cellulosic fibers for biocomposites. In: Green biorenewable biocomposites. Apple Academic Press, pp 365–384. https://doi.org/10.1201/b18092
Barleany DR, Heriyanto H, Alwan H et al (2022) Effect of starch and chitosan addition on swelling properties of neutralized poly(acrylic acid)-based superabsorbent hydrogels prepared by using γ-irradiation technique. Atom Indones 48:99. https://doi.org/10.17146/aij.2022.1171
Rodrigues FHA, Fajardo AR, Pereira AGB et al (2012) Chitosan-graft-poly(acrylic acid)/rice husk ash based superabsorbent hydrogel composite: preparation and characterization. J Polym Res 19:1. https://doi.org/10.1007/s10965-012-0001-8
Oladipo AA (2011) Synthesis and characterization of modified chitosan-based novel superabsorbent hydrogel: swelling and dye adsorption behaviorle. MSc Thesis. http://i-rep.emu.edu.tr:8080/xmlui/bitstream/handle/11129/228/Oladipo.pdf?sequence=1. Accessed 12 March 2024
Ma G, Ran F, Yang Q et al (2015) Eco-friendly superabsorbent composite based on sodium alginate and organo-loess with high swelling properties. RSC Adv 5:53819–53828. https://doi.org/10.1039/C5RA07206A
Bradley RS (2015) Loess. In: Paleoclimatology: reconstructing climates of the quaternary. Elsevier, pp 279–290. https://doi.org/10.1016/B978-0-12-386913-5.00007-7
Roberts HM, Muhs DR, Bettis EA (2013) LOESS RECORDS | North America. In: Encyclopedia of quaternary science. Elsevier, pp 620–628. https://doi.org/10.1016/B978-0-444-53643-3.00159-X
Li Y, Shi W, Aydin A et al (2020) Loess genesis and worldwide distribution. Earth-Sci Rev 201:102947. https://doi.org/10.1016/j.earscirev.2019.102947
Song B, Liang H, Sun R et al (2020) Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int J Biol Macromol 144:219–230. https://doi.org/10.1016/j.ijbiomac.2019.12.082
Mazloom N, Khorassani R, Zohury GH et al (2020) Lignin-based hydrogel alleviates drought stress in maize. Environ Exp Bot 175:104055. https://doi.org/10.1016/j.envexpbot.2020.104055
Wu L, Huang S, Zheng J et al (2019) Synthesis and characterization of biomass lignin-based PVA super-absorbent hydrogel. Int J Biol Macromol 140:538–545. https://doi.org/10.1016/j.ijbiomac.2019.08.142
Yang F, Li G, He Y-G et al (2009) Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym 78:95–99. https://doi.org/10.1016/j.carbpol.2009.04.004
Mignon A, De Belie N, Dubruel P, Van Vlierberghe S (2019) Superabsorbent polymers: a review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur Polym J 117:165–178. https://doi.org/10.1016/j.eurpolymj.2019.04.054
Ali A, Ahmed S (2018) Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J Agric Food Chem 66:6940–6967. https://doi.org/10.1021/acs.jafc.8b01052
Godiya CB, Cheng X, Li D et al (2019) Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J Hazard Mater 364:28–38. https://doi.org/10.1016/j.jhazmat.2018.09.076
Panão CO, Campos ELS, Lima HHC et al (2019) Ultra-absorbent hybrid hydrogel based on alginate and SiO2 microspheres: a high-water-content system for removal of methylene blue. J Mol Liq 276:204–213. https://doi.org/10.1016/j.molliq.2018.11.157
Chen M, Shen Y, Xu L et al (2020) Synthesis of a super-absorbent nanocomposite hydrogel based on vinyl hybrid silica nanospheres and its properties. RSC Adv 10:41022–41031. https://doi.org/10.1039/D0RA07074B
Kim HJ, Koo JM, Kim SH et al (2017) Synthesis of super absorbent polymer using citric acid as a bio-based monomer. Polym Degrad Stab 144:128–136. https://doi.org/10.1016/j.polymdegradstab.2017.07.031
Teleky B-E, Vodnar D (2019) Biomass-derived production of itaconic acid as a building block in specialty polymers. Polymers (Basel) 11:1035. https://doi.org/10.3390/polym11061035
Enawgaw H, Tesfaye T, Yilma KT, Limeneh DY (2021) Synthesis of a cellulose-Co-AMPS hydrogel for personal hygiene applications using cellulose extracted from corncobs. Gels 7:236. https://doi.org/10.3390/gels7040236
Phan TTM, Ho VC, Pham NL (2020) A study on synthesis and properties of SAPs based on carboxymethyl cellulose. Vietnam J Sci Technol Eng 62:26–32. https://doi.org/10.31276/VJSTE.62(3).26-32
Abou-Baker NH, Ouis M, Abd-Eladl M, Ibrahim MM (2020) Transformation of Lignocellulosic biomass to cellulose-based hydrogel and agriglass to improve beans yield. Waste Biomass Valoriz 11:3537–3551. https://doi.org/10.1007/s12649-019-00699-6
Liu T, Qian L, Li B et al (2013) Homogeneous synthesis of chitin-based acrylate superabsorbents in NaOH/urea solution. Carbohydr Polym 94:261–271. https://doi.org/10.1016/j.carbpol.2013.01.010
Deng F, Luo XB, Ding L, Luo SL (2019) Application of nanomaterials and nanotechnology in the reutilization of metal ion from wastewater. In: Nanomaterials for the removal of pollutants and resource reutilization. Elsevier, pp 149–178. https://doi.org/10.1016/B978-0-12-814837-2.00005-6
Guan H, Li J, Zhang B, Yu X (2017) Synthesis, properties, and humidity resistance enhancement of biodegradable cellulose-containing superabsorbent polymer. J Polym 2017:1–8. https://doi.org/10.1155/2017/3134681
Prevot V, Bourgeat-Lami E (2020) Recent advances in layered double hydroxide/polymer latex nanocomposites: from assembly to in situ formation. In: Layered double hydroxide polymer nanocomposites. Elsevier, pp 461–495. https://doi.org/10.1016/B978-0-08-101903-0.00011-8
Omura T, Suzuki T, Minami H (2021) Preparation of salt-responsive hollow hydrophilic polymer particles by inverse suspension polymerization. Langmuir 37:9371–9377. https://doi.org/10.1021/acs.langmuir.1c00834
Mayoux C, Dandurand J, Ricard A, Lacabanne C (2000) Inverse suspension polymerization of sodium acrylate: synthesis and characterization. J Appl Polym Sci 77:2621–2630. https://doi.org/10.1002/1097-4628(20000919)77:12%3c2621::AID-APP90%3e3.0.CO;2-X
Takeuchi S, Cesari A, Soma S et al (2023) Preparation of ultrasmall cyclodextrin nanogels by an inverse emulsion method using a cationic surfactant. Chem Commun 59:4071–4074. https://doi.org/10.1039/D3CC00523B
Ma J, Faqir Y, Chai Y et al (2023) Chitosan microspheres-based controlled release nitrogen fertilizers enhance the growth, antioxidant, and metabolite contents of Chinese cabbage. Sci Hortic (Amst) 308:111542. https://doi.org/10.1016/j.scienta.2022.111542
Li J, Zhang K, Zhang M et al (2018) Fabrication of a fast-swelling superabsorbent resin by inverse suspension polymerization. J Appl Polym Sci 135:46142. https://doi.org/10.1002/app.46142
Mudiyanselage TK, Neckers DC (2008) Highly absorbing superabsorbent polymer. J Polym Sci Part A Polym Chem 46:1357–1364. https://doi.org/10.1002/pola.22476
Sand A, Shin N-J, Nam H-G, Kwark Y-J (2021) Effects of reaction parameters on water absorption of poly(itaconic acid) superabsorbent particles synthesized by inverse suspension polymerization. Fibers Polym 22:898–903. https://doi.org/10.1007/s12221-021-0459-2
Kiatkamjornwong S, Chomsaksakul W, Sonsuk M (2000) Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiat Phys Chem 59:413–427. https://doi.org/10.1016/S0969-806X(00)00297-8
Azad MM, Sandros MG (2016) Microwave-assisted polymerization: superabsorbent polymer with improved properties. J Appl Polym Sci. https://doi.org/10.1002/app.43325
Azad MM, Sandros MG (2016) Microwave-assisted polymerization: Inert addition and surface coating of superabsorbent polymer for improved physical properties. J Appl Polym Sci. https://doi.org/10.1002/app.43990
Elsaeed SM, Zaki EG, Ibrahim TM et al (2021) Biochar grafted on CMC-terpolymer by green microwave route for sustainable agriculture. Agriculture 11:350. https://doi.org/10.3390/agriculture11040350
Ghasri M, Bouhendi H, Kabiri K et al (2019) Superabsorbent polymers achieved by surface cross linking of poly(sodium acrylate) using microwave method. Iran Polym J 28:539–548. https://doi.org/10.1007/s13726-019-00722-6
Gao C, Yan D (2011) Synthesis of hyperbranched polymers via polymerization of asymmetric monomer pairs. In: Hyperbranched polymers. Wiley, Hoboken, pp 107–138. https://doi.org/10.1002/9780470929001.ch4
Kalita P, Pegu CD, Dutta P, Baruah PK (2014) Room temperature solvent free aza-Michael reactions over nano-cage mesoporous materials. J Mol Catal A Chem 394:145–150. https://doi.org/10.1016/j.molcata.2014.06.031
Mather BD, Viswanathan K, Miller KM, Long TE (2006) Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci 31:487–531. https://doi.org/10.1016/j.progpolymsci.2006.03.001
Naga N, Fujioka S, Inose D et al (2020) Synthesis and properties of porous polymers synthesized by Michael addition reactions of multi-functional acrylate, diamine, and dithiol compounds. RSC Adv 10:60–69. https://doi.org/10.1039/C9RA09684A
Sashiwa H, Yamamori N, Ichinose Y et al (2003) Chemical modification of chitosan, 17. Macromol Biosci 3:231–233. https://doi.org/10.1002/mabi.200390029
Sashiwa H, Yamamori N, Ichinose Y et al (2003) Michael reaction of chitosan with various acryl reagents in water. Biomacromol 4:1250–1254. https://doi.org/10.1021/bm030022o
Fujita M, Iizuka Y, Miyake A (2017) Thermal and kinetic analyses on Michael addition reaction of acrylic acid. J Therm Anal Calorim 128:1227–1233. https://doi.org/10.1007/s10973-016-5985-6
Huang S, Sinha J, Podgórski M et al (2018) Mechanistic modeling of the Thiol-Michael addition polymerization kinetics: structural effects of the thiol and vinyl monomers. Macromolecules 51:5979–5988. https://doi.org/10.1021/acs.macromol.8b01264
Yun K-K, Kim K-K, Choi W, Yeon J (2017) Hygral behavior of superabsorbent polymers with various particle sizes and cross-linking densities. Polymers (Basel) 9:600. https://doi.org/10.3390/polym9110600
Che Ani N, Jamari SS, Wan Yaacob WSN (2016) Effect of cross-linker concentration on the synthesis and swelling behaviour of superabsorbent polymers (SAP) using graft polymerization techniques. Key Eng Mater 719:62–66. https://doi.org/10.4028/www.scientific.net/KEM.719.62
Ott MW, Herbert H, Graf M, Biesalski M (2016) Cellulose-graft-polystyrene bottle-brush copolymers by homogeneous RAFT polymerization of soluble cellulose macro-CTAs and “CTA-shuttled” R-group approach. Polymer (Guildf) 98:505–515. https://doi.org/10.1016/j.polymer.2016.05.006
Singh V, Kumar P, Sanghi R (2012) Use of microwave irradiation in the grafting modification of the polysaccharides—a review. Prog Polym Sci 37:340–364. https://doi.org/10.1016/j.progpolymsci.2011.07.005
Menon S, Deepthi VM, Sailaja RRNAGS (2014) Study on microwave assisted synthesis of biodegradable guar gum grafted acrylic acid superabsorbent nanocomposites. Indian J Adv Chem Sci 2:76–83
Kim S, Kim M, Koh W-G (2021) Preparation of surface-reinforced superabsorbent polymer hydrogel microspheres via incorporation of in situ synthesized silver nanoparticles. Polymers (Basel) 13:902. https://doi.org/10.3390/polym13060902
Lai J, Bi Y, Zhou Y et al (2023) Synthesis and characterization of a new super absorbent polymer (SAP) via the use of low-grade kaolin through inverse suspension polymerization. Constr Build Mater 363:129849. https://doi.org/10.1016/j.conbuildmat.2022.129849
Qureshi MA, Nishat N, Jadoun S, Ansari MZ (2020) Polysaccharide based superabsorbent hydrogels and their methods of synthesis: a review. Carbohydr Polym Technol Appl 1:100014. https://doi.org/10.1016/j.carpta.2020.100014
Omidian H, Zohuriaan-Mehr MJ, Bouhendi H (2003) Polymerization of sodium acrylate in inverse-suspension stabilized by sorbitan fatty esters. Eur Polym J 39:1013–1018. https://doi.org/10.1016/S0014-3057(02)00352-X
Tanan W, Panpinit S, Saengsuwan S (2021) Comparison of microwave-assisted and thermal-heated synthesis of P(HEMA-co-AM)/PVA interpenetrating polymer network (IPN) hydrogels for Pb(II) removal from aqueous solution: characterization, adsorption and kinetic study. Eur Polym J 143:110193. https://doi.org/10.1016/j.eurpolymj.2020.110193
Abidin AZ, Putra RP, Izzati AUN, Christian Y (2021) Design and performance evaluation of a superabsorbent polymer-based dryer for medicinal plants. J Food Process Preserv. https://doi.org/10.1111/jfpp.15988
Wiesbrock F, Hoogenboom R, Schubert US (2004) Microwave-assisted polymer synthesis: state-of-the-art and future perspectives. Macromol Rapid Commun 25:1739–1764. https://doi.org/10.1002/marc.200400313
Lai H, Zhang J, Xing F, Xiao P (2020) Recent advances in light-regulated non-radical polymerisations. Chem Soc Rev 49:1867–1886. https://doi.org/10.1039/C9CS00731H
Rajkumar K, Muthukumar M (2017) Response surface optimization of electro-oxidation process for the treatment of C.I. Reactive Yellow 186 dye: reaction pathways. Appl Water Sci 7:637–652. https://doi.org/10.1007/s13201-015-0276-0
Sirichan T, Kijpatanasilp I, Asadatorn N, Assatarakul K (2022) Optimization of ultrasound extraction of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason Sonochem 83:105916. https://doi.org/10.1016/j.ultsonch.2022.105916
Kim H-C, Kwon Y-R, Kim J-S et al (2022) Computational approach to the surface-crosslinking process of superabsorbent polymer via central composite design. Polymers (Basel) 14:3842. https://doi.org/10.3390/polym14183842
Yousefi M, Nabizadeh R, Alimohammadi M et al (2019) Performance of granular ferric hydroxide process for removal of humic acid substances from aqueous solution based on experimental design and response surface methodology. MethodsX 6:35–42. https://doi.org/10.1016/j.mex.2018.12.010
Savaskan Yilmaz S, Yildirim N, Misir M et al (2020) Synthesis, characterization of a new polyacrylic acid superabsorbent, some heavy metal ion sorption, the adsorption isotherms, and quantum chemical investigation. Materials (Basel) 13:4390. https://doi.org/10.3390/ma13194390
Zhang C, Yu S, Wang F et al (2022) Density functional theory analysis of the copolymerization of cyclopropenone with ethylene using a palladium catalyst. Polymers (Basel) 14:5273. https://doi.org/10.3390/polym14235273
Eslick JC, Ye Q, Park J et al (2009) A computational molecular design framework for crosslinked polymer networks. Comput Chem Eng 33:954–963. https://doi.org/10.1016/j.compchemeng.2008.09.019
Yarolimek MR, Bookbinder HR, Coia BM, Kennemur JG (2021) Ring-opening metathesis polymerization of δ-pinene: well-defined polyolefins from pine sap. ACS Macro Lett 10:760–766. https://doi.org/10.1021/acsmacrolett.1c00284
Romero Rodriguez C, Chaves Figueiredo S, Schlangen E, Snoeck D (2018) Modeling water absorption in cement-based composites with SAP additions. In: Computational modelling of concrete structures. CRC Press, London, pp 295–304. https://doi.org/10.1201/9781315182964-37
Sweijen T, van Duijn CJ, Hassanizadeh SM (2017) A model for diffusion of water into a swelling particle with a free boundary: application to a super absorbent polymer particle. Chem Eng Sci 172:407–413. https://doi.org/10.1016/j.ces.2017.06.045
Wu S, Kondo Y, Kakimoto M et al (2019) Machine-learning-assisted discovery of polymers with high thermal conductivity using a molecular design algorithm. npj Comput Mater 5:66. https://doi.org/10.1038/s41524-019-0203-2
Pruksawan S, Wang F (2023) Modeling of toughening effect in rigid particulate-filled polymer composites by artificial intelligence: a review. Adv Compos Mater 32:250–267. https://doi.org/10.1080/09243046.2022.2083304
Song L, Zhang Y, Zhan J et al (2022) Interfacial thermal resistance in polymer composites: a molecular dynamic perspective. Mol Simul 48:902–925. https://doi.org/10.1080/08927022.2022.2071874
Martins VG, Costa JAV, Prentice C (2018) Water-uptake properties of a fish protein-based superabsorbent hydrogel chemically modified with ethanol. Polímeros 28:196–204. https://doi.org/10.1590/0104-1428.0117
Fořt J, Migas P, Černý R (2020) Effect of absorptivity of superabsorbent polymers on design of cement mortars. Materials (Basel) 13:5503. https://doi.org/10.3390/ma13235503
Ismaeilimoghadam S, Jonoobi M, Hamzeh Y, Danti S (2022) Effect of nanocellulose types on microporous acrylic acid/sodium alginate super absorbent polymers. J Funct Biomater 13:273. https://doi.org/10.3390/jfb13040273
Yang M, Dong W, Cheng R et al (2022) Effect of highly efficient substrate modifier, super-absorbent polymer, on the performance of the green roof. Sci Total Environ 806:150638. https://doi.org/10.1016/j.scitotenv.2021.150638
Peng G, Xu S, Peng Y et al (2008) A new amphoteric superabsorbent hydrogel based on sodium starch sulfate. Bioresour Technol 99:444–447. https://doi.org/10.1016/j.biortech.2007.01.018
Zhao C, Zhang M, Liu Z et al (2019) Salt-tolerant superabsorbent polymer with high capacity of water-nutrient retention derived from sulfamic acid-modified starch. ACS Omega 4:5923–5930. https://doi.org/10.1021/acsomega.9b00486
Adair A, Klinpituksa P, Kaesaman A (2017) Influences of neutralization of superabsorbent hydrogel from hydroxyethyl cellulose on water swelling capacities. AIP Conf Proc 1868:020012. https://doi.org/10.1063/1.4995098
Wang C, Bu Y, Guo S et al (2019) Self-healing cement composite: amine- and ammonium-based pH-sensitive superabsorbent polymers. Cem Concr Compos 96:154–162. https://doi.org/10.1016/j.cemconcomp.2018.11.023
Constable P (2009) Clinical acid-base chemistry. In: Critical care nephrology. Elsevier, pp 581–586. https://doi.org/10.1016/B978-1-4160-4252-5.50116-7
Mehta AW, Emmett M (2009) Approach to acid-base disorders. In: Primer on kidney diseases. Elsevier, pp 98–107. https://doi.org/10.1016/B978-1-4160-5185-5.00011-0
Kida K, Okita M, Fujita K et al (2013) Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm. https://doi.org/10.1039/c2ce26847g
Lyu Z, Shen A, Mo S et al (2020) Life-cycle crack resistance and micro characteristics of internally cured concrete with superabsorbent polymers. Constr Build Mater 259:119794. https://doi.org/10.1016/j.conbuildmat.2020.119794
Mogale R, Akpomie KG, Conradie J, Langner EHG (2022) Dye adsorption of aluminium- and zirconium-based metal organic frameworks with azobenzene dicarboxylate linkers. J Environ Manag 304:114166. https://doi.org/10.1016/j.jenvman.2021.114166
Kang S-H, Hong S-G, Moon J (2017) Absorption kinetics of superabsorbent polymers (SAP) in various cement-based solutions. Cem Concr Res 97:73–83. https://doi.org/10.1016/j.cemconres.2017.03.009
Lyu Z, Shen A, He Z et al (2022) Absorption characteristics and shrinkage mitigation of superabsorbent polymers in pavement concrete. Int J Pavement Eng 23:270–284. https://doi.org/10.1080/10298436.2020.1742334
Lim BR, Mazlan SNA, Ghazali S et al (2020) Effect of nano-fibril cellulose (NFC) filler towards the swelling and diffusion behavior of superabsorbent polymer composite. IOP Conf Ser Mater Sci Eng 991:012114. https://doi.org/10.1088/1757-899X/991/1/012114
Pourjavadi A, Farhadpour B, Seidi F (2008) Synthesis and investigation of swelling behavior of grafted alginate/alumina superabsorbent composite. Starch Stärke 60:457–466. https://doi.org/10.1002/star.200800208
Pajerski W, Ochonska D, Brzychczy-Wloch M et al (2019) Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J Nanoparticle Res 21:186. https://doi.org/10.1007/s11051-019-4617-z
Petrova MI, Lievens E, Malik S et al (2015) Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. https://doi.org/10.3389/fphys.2015.00081
Pandey M, Choudhury H, Abdul-Aziz A et al (2020) Promising drug delivery approaches to treat microbial infections in the vagina: a recent update. Polymers (Basel) 13:26. https://doi.org/10.3390/polym13010026
Tachedjian G, O’Hanlon DE, Ravel J (2018) The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome 6:29. https://doi.org/10.1186/s40168-018-0418-3
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA (2017) The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168:782–792. https://doi.org/10.1016/j.resmic.2017.04.001
Boyanova L, Marteva-Proevska Y, Gergova R, Markovska R (2021) Gardnerella vaginalis in urinary tract infections, are men spared? Anaerobe 72:102438. https://doi.org/10.1016/j.anaerobe.2021.102438
Unver T, Erenler A, Melekoglu R et al (2020) Antifungal activity of microbial chondroitin sulfate against candida albicans. Med Sci Int Med J. https://doi.org/10.5455/medscience.2019.08.9208
Morrill S, Gilbert NM, Lewis AL (2020) Gardnerella vaginalis as a cause of bacterial vaginosis: appraisal of the evidence from in vivo models. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.00168
McCormick JK, Yarwood JM, Schlievert PM (2001) Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55:77–104. https://doi.org/10.1146/annurev.micro.55.1.77
Schlievert PM, Davis CC (2020) Device-associated menstrual toxic shock syndrome. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00032-19
Takia L, Lodha R (2023) Toxic shock syndrome: a diagnostic and therapeutic challenge! Indian J Pediatr. https://doi.org/10.1007/s12098-023-04478-z
Contou D, Colin G, Travert B et al (2022) Menstrual toxic shock syndrome: a French nationwide multicenter retrospective study. Clin Infect Dis 74:246–253. https://doi.org/10.1093/cid/ciab378
Berger S, Kunerl A, Wasmuth S et al (2019) Menstrual toxic shock syndrome: case report and systematic review of the literature. Lancet Infect Dis 19:e313–e321. https://doi.org/10.1016/S1473-3099(19)30041-6
Foster J, Montgomery P (2021) A study of environmentally friendly menstrual absorbents in the context of social change for adolescent girls in low- and middle-income countries. Int J Environ Res Public Health 18:9766. https://doi.org/10.3390/ijerph18189766
Sun Y, Li C, Yan Y et al (2022) Effects of hemp sanitary pads on the vaginal microecology. Comput Math Methods Med 2022:1–6. https://doi.org/10.1155/2022/4435722
Benhabiles MS, Salah R, Lounici H et al (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29:48–56. https://doi.org/10.1016/j.foodhyd.2012.02.013
Lopez-Moya F, Suarez-Fernandez M, Lopez-Llorca L (2019) Molecular mechanisms of chitosan interactions with fungi and plants. Int J Mol Sci 20:332. https://doi.org/10.3390/ijms20020332
Jøraholmen MW, Bhargava A, Julin K et al (2020) The antimicrobial properties of chitosan can be tailored by formulation. Mar Drugs 18:96. https://doi.org/10.3390/md18020096
Andersen T, Bleher S, Eide Flaten G et al (2015) Chitosan in mucoadhesive drug delivery: focus on local vaginal therapy. Mar Drugs 13:222–236. https://doi.org/10.3390/md13010222
(2023) BFree Cup. In: BFree. https://bfreecup.com/. Accessed 12 Apr 2023
Pillai OM, Sundaramoorthy S (2022) Development of photocatalytic self-cleaning textiles using tin oxide and titanium dioxide nanoparticles mixture. J Text Inst. https://doi.org/10.1080/00405000.2022.2062859
Tania IS, Ali M (2021) Coating of ZnO nanoparticle on cotton fabric to create a functional textile with enhanced mechanical properties. Polymers (Basel) 13:2701. https://doi.org/10.3390/polym13162701
Tania IS, Ali M (2021) Effect of the coating of zinc oxide (ZnO) nanoparticles with binder on the functional and mechanical properties of cotton fabric. Mater Today Proc 38:2607–2611. https://doi.org/10.1016/j.matpr.2020.08.171
Mehravani B, Ribeiro A, Zille A (2021) Gold nanoparticles synthesis and antimicrobial effect on fibrous materials. Nanomaterials 11:1067. https://doi.org/10.3390/nano11051067
Emam HE, Zahran MK (2015) Ag0 nanoparticles containing cotton fabric: synthesis, characterization, color data and antibacterial action. Int J Biol Macromol 75:106–114. https://doi.org/10.1016/j.ijbiomac.2014.12.050
Periyasamy AP, Venkataraman M, Kremenakova D et al (2020) Progress in sol-gel technology for the coatings of fabrics. Materials (Basel) 13:1838. https://doi.org/10.3390/ma13081838
Banerjee S, Pillai SC, Falaras P et al (2014) New insights into the mechanism of visible light photocatalysis. J Phys Chem Lett 5:2543–2554. https://doi.org/10.1021/jz501030x
Kavitha Sankar PC, Ramakrishnan R, Rosemary MJ (2016) Biological evaluation of nanosilver incorporated cellulose pulp for hygiene products. Mater Sci Eng C 61:631–637. https://doi.org/10.1016/j.msec.2015.12.072
Ben AI, Hemmami H, Laouini SE et al (2023) Biosynthesis MgO and ZnO nanoparticles using chitosan extracted from Pimelia Payraudi Latreille for antibacterial applications. World J Microbiol Biotechnol 39:19. https://doi.org/10.1007/s11274-022-03464-5
Maghsoudi S, Nasiri Pour P, Ebrahimnejad H et al (2022) Decoration of cotton fiber with biosynthesized Ag/ZnO nanocomposite for durable antibacterial textile. J Nat Fibers 19:8033–8043. https://doi.org/10.1080/15440478.2021.1958433
Acknowledgements
This work was supported by the University of the Free State postdoctoral fellowship program.
Funding
Open access funding provided by University of the Free State.
Author information
Authors and Affiliations
Contributions
RM was involved in conceptualization, writing, and reviewing. MS-S, EE, KDW, and HGV helped with writing and reviewing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing interests for the work reported in this paper.
Ethical approval
This work does not require any ethical statement.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mogale, R., Schutte-Smith, M., Erasmus, E. et al. Toward sustainable menstrual health management: focus on super absorbent polymers. J Mater Sci 59, 6138–6168 (2024). https://doi.org/10.1007/s10853-024-09519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09519-2