Abstract
Nanoporous metals can serve in unique functional applications due to their high surface area, and they are often made through dealloying, which removes a sacrificial element through chemical etching and leaves a nanoporous matrix of the remaining element(s). Several variations on this technique allow for a wider variety of metals and alloys to be processed, but simple, scalable, and reliable production of nanoporous devices is still not a reality using the majority of techniques and alloy systems. Oxide reduction may provide a suitable alternative, and in this work, nanoporous Cu was produced via oxide reduction in the Cu–CuO system. This method uses mechanical milling to create a metal matrix composite powder primarily composed of CuO that is reduced under hydrogen to leave pure Cu behind. It requires no harsh chemicals, and it uses modest temperatures and short reaction times. Furthermore, it is fully compatible with traditional powder metallurgy, making it readily scalable. Powder compacts were also processed to track the geometric changes associated with each composition and demonstrate that device creation is feasible, even with the basic process applied here. This work reveals important parameters associated with these materials by incorporating a wide range of milling times and compositions, with shorter milling times and higher oxide concentrations providing the finest pore sizes, the highest porosity, and the greatest simplicity.
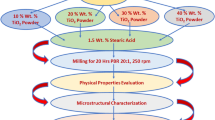
Graphical abstract











Similar content being viewed by others
Data and Code Availability
All data are available upon request.
References
Ding Y, Chen M (2009) Nanoporous metals for catalytic and optical applications. MRS Bull 34(8):569–576
Lu L (2019) Nanoporous noble metal-based alloys: a review on synthesis and applications to electrocatalysis and electrochemical sensing. Microchim Acta 186(9):1–21
Chen L-Y, Yu J-S, Fujita T, Chen M-W (2009) Nanoporous copper with tunable nanoporosity for SERS applications. Adv Func Mater 19(8):1221–1226
Sattayasamitsathit S, Thavarungkul P, Thammakhet C, Limbut W, Numnuam A, Buranachai C, Kanatharana P (2009) Fabrication of nanoporous copper film for electrochemical detection of glucose. Electroanal 21(21):2371–2377
Luo M, Peng W, Zhao Y, Lan J, Peng M, Han J, Tan Y (2021) Dilute molybdenum atoms embedded in hierarchical nanoporous copper accelerate the hydrogen evolution reaction. Scripta Mater 191:56–61
Lv JJ, Jouny M, Luc W, Zhu W, Zhu JJ, Jiao F (2018) A highly porous copper electrocatalyst for carbon dioxide reduction. Adv Mater 30(49):1803111
Li M, Liu J, Wang C, Liu Y, Sun Y, Qin C, Liu S (2022) Controllable nanoporous copper synthesized by dealloying metallic glasses: New insights into the tuning pore structure and applications. Chem Eng J 427:130861
Chen F, Chen X, Zou L, Yao Y, Lin Y, Shen Q, Zhang L (2016) Fabrication and mechanical behavior of bulk nanoporous Cu via chemical de-alloying of Cu–Al alloys. Mater Sci Eng: A 660:241–250
Qi Z, Zhao C, Wang X, Lin J, Shao W, Zhang Z, Bian X (2009) Formation and characterization of monolithic nanoporous copper by chemical dealloying of Al− Cu alloys. J Phys Chem C 113(16):6694–6698
Tuan NT, Park J, Lee J, Gwak J, Lee D (2014) Synthesis of nanoporous Cu films by dealloying of electrochemically deposited Cu–Zn alloy films. Corros Sci 80:7–11
Lu H-B, Li Y, Wang F-H (2007) Synthesis of porous copper from nanocrystalline two-phase Cu–Zr film by dealloying. Scripta Mater 56:165–168
Dan Z, Qin F, Sugawara Y, Muto I, Hara N (2013) Effects of the initial microstructure of Ti–Cu alloys on final nanoporous copper via dealloying. J Alloys Compd 557:166–171
Dan Z, Qin F, Yamaura S-I, Sugawara Y, Muto I, Hara N (2013) Dealloying behavior of amorphous binary Ti–Cu alloys in hydrofluoric acid solutions at various temperatures. J Alloys Compd 581:567–572
Keir D, Pryor M (1980) The dealloying of copper-manganese alloys. J Electrochem Soc 127(10):2138–2144
Min U-S, Li JC (1994) The microstructure and dealloying kinetics of a Cu-Mn alloy. J Mater Res 9(11):2878–2883
Hayes J, Hodge A, Biener J, Hamza A, Sieradzki K (2006) Monolithic nanoporous copper by dealloying Mn–Cu. J Mater Res 21(10):2611–2616
Atwater MA, Darling KA, Tschopp MA (2014) Towards reaching the theoretical limit of porosity in solid state metal foams: intraparticle expansion as a primary and additive means to create porosity. Adv Eng Mater 16(2):190–195
Atwater MA, Darling KA, Tschopp MA (2016) Solid-state foaming by oxide reduction and expansion: tailoring the foamed metal microstructure in the Cu–CuO system with oxide content and annealing conditions. Adv Eng Mater 18(1):83–95
Atwater M, Corcoran S (2023) Using powder metallurgy and oxide reduction to produce eco-friendly bulk nanoporous nickel. Adv Eng Mater. https://doi.org/10.1002/adem.202201629
Rodriguez JA, Kim JY, Hanson JC, Pérez M, Frenkel AI (2003) Reduction of CuO in H2: in situ time-resolved XRD studies. Catal Lett 85(3):247–254
Rukini A, Rhamdhani M, Brooks G, Van den Bulck A (2022) Metals production and metal oxides reduction using hydrogen: a review. J Sustain Metall 8(1):1–24
Pawar SM, Kim J, Inamdar AI, Woo H, Jo Y, Pawar BS, Im H (2016) Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci Rep 6(1):21310
McCormick P, Froes F (1998) The fundamentals of mechanochemical processing. JOM 50:61–65
Khayati G, Nourafkan E, Karimi G, Moradgholi J (2013) Synthesis of cuprous oxide nanoparticles by mechanochemical oxidation of copper in high planetary energy ball mill. Adv Powder Technol 24(1):301–305
Tokumitsu K (1997) Reduction of metal oxides by mechanical alloying method. Solid State Ionics 101:25–31
Suryanarayana C (2001) Mechanical alloying and milling. Prog Mater Sci 46:1–184
Unutulmazsoy Y, Cancellieri C, Lin L, Jeurgens LP (2022) Reduction of thermally grown single-phase CuO and Cu2O thin films by in-situ time-resolved XRD. Appl Surf Sci 588:152896
Kim JY, Rodriguez JA, Hanson JC, Frenkel AI, Lee PL (2003) Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J Am Chem Soc 125(35):10684–10692
Nes E, Ryum N, Hunderi O (1985) On the Zener drag. Acta Metall 33(1):11–22
German RM (2010) Coarsening in sintering: grain shape distribution, grain size distribution, and grain growth kinetics in solid-pore systems. Cr Rev Sol State 35(4):263–305
Cullity BD, Stock SR (2001) Elements of X-Ray diffraction. Prentice Hall, USA
Erlebacher J, Sieradzki K (2003) Pattern formation during dealloying. Scripta Mater 49(10):991–996
Erlebacher J, Aziz MJ, Karma A, Dimitrov N, Sieradzki K (2001) Evolution of nanoporosity in dealloying. Nature 410(6827):450–453
McCue I, Benn E, Gaskey B, Erlebacher J (2016) Dealloying and dealloyed materials. Ann Rev Mater Res 46:263–286
McCue I, Gaskey B, Geslin P-A, Karma A, Erlebacher J (2016) Kinetics and morphological evolution of liquid metal dealloying. Acta Mater 115:10–23
Geslin P-A, McCue I, Gaskey B, Erlebacher J, Karma A (2015) Topology-generating interfacial pattern formation during liquid metal dealloying. Nat Commun 6:8887
McCue I, Demkowicz MJ (2017) Alloy design criteria for solid metal dealloying of thin films. JOM 69(11):2199–2205
Zhao C, Kisslinger K, Huang X, Lu M, Camino F, Lin C-H, Ravel B (2019) Bi-continuous pattern formation in thin films via solid-state interfacial dealloying studied by multimodal characterization. Mater Horiz 6(10):1991–2002
Han J, Li C, Lu Z, Wang H, Wang Z, Watanabe K, Chen M (2019) Vapor phase dealloying: a versatile approach for fabricating 3D porous materials. Acta Mater 163:161–172
Lu Z, Li C, Han J, Zhang F, Liu P, Wang H, Hirata A (2018) Three-dimensional bicontinuous nanoporous materials by vapor phase dealloying. Nat Commun 9(1):276
Pinna A, Pia G, Casula MF, Delogu F, Sogne E, Falqui A, Pilia L (2021) Fabrication of nanoporous Al by vapor-phase dealloying: morphology features, mechanical properties and model predictions. Appl Sci 11(14):6639
Scandura G, Kumari P, Palmisano G, Karanikolos GN, Orwa J, Dumée LF (2023) Nanoporous dealloyed metal materials processing and applications—a review. Ind Eng Chem Res 62(4):1736–1763
Sun L, Chien C-L, Searson PC (2004) Fabrication of nanoporous nickel by electrochemical dealloying. Chem Mater 16(16):3125–3129
Detsi E, Van De Schootbrugge M, Punzhin S, Onck P, De Hosson J (2011) On tuning the morphology of nanoporous gold. Scripta Mater 64(4):319–322
Wang Y, Wang Y, Zhang C, Kou T, Zhang Z (2012) Tuning the ligament/channel size of nanoporous copper by temperature control. CrystEngComm 14(24):8352–8356
Liu W, Zhang S, Li N, Zheng J, Xing Y (2011) A facile one-pot route to fabricate nanoporous copper with controlled hierarchical pore size distributions through chemical dealloying of Al–Cu alloy in an alkaline solution. Microporous Mesoporous Mater 138(1–3):1–7
Wang N, Pan Y, Wu S (2018) Relationship between dealloying conditions and coarsening behaviors of nanoporous copper fabricated by dealloying Cu-Ce metallic glasses. J Mater Sci Technol 34(7):1162–1171
Jin HJ, Weissmüller J (2010) Bulk nanoporous metal for actuation. Adv Eng Mater 12(8):714–723
Okulov I, Okulov A, Soldatov I, Luthringer B, Willumeit-Römer R, Wada T, Markmann J (2018) Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater Sci Eng: C 88:95–103
Garner S, Ruiz E, Strong J, Zavaliangos A (2014) Mechanisms of crack formation in die compacted powders during unloading and ejection: an experimental and modeling comparison between standard straight and tapered dies. Powder Technol 264:114–127
Fujita T (2017) Hierarchical nanoporous metals as a path toward the ultimate three-dimensional functionality. Sci Technol Adv Mater 18(1):724–740
Miyanaji H, Ma D, Atwater MA, Darling KA, Hammond VH, Williams CB (2020) Binder jetting additive manufacturing of copper foam structures. Addit Manufact 32:100960
Chuang A, Erlebacher J (2020) Challenges and opportunities for integrating dealloying methods into additive manufacturing. Materials 13(17):3706
Sun Y, Ren Y (2015) New preparation method of porous copper powder through vacuum dealloying. Vacuum 122:215–217
Seker E, Reed ML, Begley MR (2009) Nanoporous gold: fabrication, characterization, and applications. Materials 2(4):2188–2215
Nyce GW, Hayes JR, Hamza AV, Satcher JH (2007) Synthesis and characterization of hierarchical porous gold materials. Chem Mater 19(3):344–346
Cherevko S, Chung C-H (2011) Direct electrodeposition of nanoporous gold with controlled multimodal pore size distribution. Electrochem Commun 13(1):16–19
Fujita T, Qian LH, Inoke K, Erlebacher J, Chen MW (2008) Three-dimensional morphology of nanoporous gold. Appl Phys Lett 92(25):251902
Van Petegem S, Brandstetter S, Maass R, Hodge AM, El-Dasher BS, Biener J, Schmitt B, Borca C, Van Swygenhoven H (2009) On the microstructure of nanoporous gold: an x-ray diffraction study. Nano Lett 9(3):1158–1163
Zielasek V, Jürgens B, Schulz C, Biener J, Biener MM, Hamza AV, Bäumer M (2006) Gold catalysts: nanoporous gold foams. Angew Chem Int Ed 45(48):8241–8244
Acknowledgements
MA is particularly grateful to Constance Ziemian and the Mechanical Engineering faculty of Bucknell University for their support during the initial stages of this investigation, and funding through the Department of Mechanical Engineering. The authors would like to thank ThermoFisher for their support in collecting the FIB sections on the 75% CuO sample. We are grateful to Hitachi HighTech for providing the FIB section images for the 90% CuO sample. MA is also grateful for the financial support of this work by the National Science Foundation [CAREER award #2035473].
Author information
Authors and Affiliations
Contributions
DJ—Investigation, Writing—Review and Editing; BB—Investigation, Writing—Review and Editing; NA—Investigation, Writing—Review and Editing; MA—Conceptualization, Methodology, Investigation, Writing—Original Draft; Supervision, Project Administration, Funding Acquisition.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not Applicable.
Additional information
Handling Editor: N. Ravishankar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, D., Boan, B., Allapatt, N. et al. Nanoporous Cu created by the reduction of CuO dispersions. J Mater Sci 58, 12635–12649 (2023). https://doi.org/10.1007/s10853-023-08817-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08817-5