Abstract
The earth-abundant transition metal manganese (Mn) has been shown to be useful to activate dinitrogen at atmospheric pressure and elevated temperature by forming bulk Mn nitrides. Mn nitrides could then be used, for example, for ammonia (NH3) synthesis in a chemical looping process by contacting nitride with gaseous hydrogen (H2). Here, we present an investigation of the morphology and local time-dependent composition of micrometer-scale Mn plates during nitridation in dinitrogen (N2) near atmospheric pressure at 700 °C. The main motivation was to obtain design data for chemical looping synthesis of NH3 and to add to the somewhat sparse literature on nitridation of Mn. The morphology and elemental compositional variation of the nitrided specimens were studied with scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), wide angle X-ray diffraction (WAXD), and mass balances. Three possible nitrogen (N) populations that may govern the Mn nitridation and later NH3 synthesis are identified. After four hours of nitridation, the N weight gain was found to be 9.4 ± 0.7 kgN to nMn−1 for the plates used here, resulting in a nitridation depth of 83 ± 8 μm.
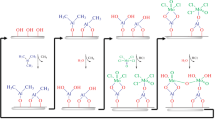
Graphical abstract

















Similar content being viewed by others
References
Aframehr WM, Huang C, Pfromm PH (2020) Chemical looping of manganese to synthesize ammonia at atmospheric pressure: sodium as promoter. Chem Eng Technol 43:2126–2133. https://doi.org/10.1002/ceat.202000154
Michalsky R, Pfromm PH (2012) An ionicity rationale to design solid phase metal nitride reactants for solar ammonia production. J Phys Chem C 116:23243–23251. https://doi.org/10.1021/jp307382r
Shalom M, Ressnig D, Yang X et al (2015) Nickel nitride as an efficient electrocatalyst for water splitting. J Mater Chem A 3:8171–8177. https://doi.org/10.1039/c5ta00078e
Sirajuddeen MMS, Banu IBS (2015) Electronic and magnetic properties of Fe(Mn)-doped Cd and Zn nitrides for spintronic applications: a first-principles study. J Mater Sci 50:1446–1456. https://doi.org/10.1007/s10853-014-8705-2
Kumar V, Roy DR (2018) Structure, bonding, stability, electronic, thermodynamic and thermoelectric properties of six different phases of indium nitride. J Mater Sci 53:8302–8313. https://doi.org/10.1007/s10853-018-2176-9
Gharavi MA, Armiento R, Alling B, Eklund P (2020) Theoretical study of the phase transitions and electronic structure of (Zr0.5, Mg0.5)N and (Hf0.5, Mg0.5)N. J Mater Sci. https://doi.org/https://doi.org/10.1007/s10853-020-05372-1
Rudziński M, Zlotnik S, Wójcik M et al (2021) Mask-free three-dimensional epitaxial growth of III-nitrides. J Mater Sci 56:558–569. https://doi.org/10.1007/s10853-020-05187-0
Juangsa FB, Aziz M (2019) Integrated system of thermochemical cycle of ammonia, nitrogen production, and power generation. Int J Hydrogen Energy 44:17525–17534. https://doi.org/10.1016/j.ijhydene.2019.05.110
Edrisi A, Mansoori Z, Dabir B (2014) Using three chemical looping reactors in ammonia production process—a novel plant configuration for a green production. Int J Hydrogen Energy 39:8271–8282. https://doi.org/10.1016/j.ijhydene.2014.03.119
Liu H, Han W, Huo C, Cen Y (2020) Development and application of wüstite-based ammonia synthesis catalysts. Catal Today 355:110–127. https://doi.org/10.1016/j.cattod.2019.10.031
Pfromm PH (2017) Towards sustainable agriculture: fossil-free ammonia. J Renew Sustain Energy 9. https://doi.org/https://doi.org/10.1063/1.4985090
Michalsky R, Parman BJ, Amanor-boadu V, Pfromm PH (2012) Solar thermochemical production of ammonia from water, air and sunlight: thermodynamic and economic analyses. Energy 42:251–260. https://doi.org/10.1016/j.energy.2012.03.062
Heidlage MG, Kezar EA, Snow KC, Pfromm PH (2017) Thermochemical synthesis of ammonia and syngas from natural gas at atmospheric pressure. 14014–14024. https://doi.org/https://doi.org/10.1021/acs.iecr.7b03173
Michalsky R, Pfromm PH (2012) Thermodynamics of metal reactants for ammonia synthesis from steam. Nitrogen Biomass Atmos Pressure 58:3203–3213. https://doi.org/10.1002/aic
Michalsky R, Avram AM, Peterson BA, et al (2015) Chemical science pressure ammonia synthesis for energy storage †. 3965–3974. https://doi.org/https://doi.org/10.1039/c5sc00789e
Chen JG, Crooks RM, Seefeldt LC, et al (2018) nitrogen transformations 6611. https://doi.org/https://doi.org/10.1126/science.aar6611
Aakko-saksa PT, Cook C, Kiviaho J, Repo T (2018) Liquid organic hydrogen carriers for transportation and storing of renewable energy—review and discussion. J Power Sources 396:803–823. https://doi.org/10.1016/j.jpowsour.2018.04.011
Michalsky R, Avram AM, Peterson BA et al (2015) Chemical looping of metal nitride catalysts: low-pressure ammonia synthesis for energy storage. Chem Sci 6:3965–3974. https://doi.org/10.1039/c5sc00789e
Singstock NR, Bartel CJ, Holder AM, Musgrave CB (2020) High-throughput analysis of materials for chemical looping processes. Adv Energy Mater 10:1–11. https://doi.org/10.1002/aenm.202000685
Abad A (2007) The use of iron oxide as oxygen carrier in a chemical-looping reactor. 86:1021–1035. https://doi.org/https://doi.org/10.1016/j.fuel.2006.09.021
Sun R, Yan J, Shen L, Bai H (2020) Performance and mechanism study of LaFeO3 for biomass chemical looping gasification. J Mater Sci 55:11151–11166. https://doi.org/10.1007/s10853-020-04890-2
Michalsky R, Pfromm PH, Steinfeld A (2015) Rational design of metal nitride redox materials for solar-driven ammonia synthesis. Interface Focus 5:1–10. https://doi.org/10.1098/rsfs.2014.0084
Lengauer W, Wiesenberger H, Mayr W et al (1997) Phase stabilities of transition metal carbides and nitrides investigated by reaction diffusion. J Chim Phys Physico-Chimie Biol 94:1020–1025. https://doi.org/10.1051/jcp/1997941020
Kral C, Lengauer W, Rafaja D, Ettmayer P (1998) Critical review on the elastic properties of transition metal carbides, nitrides and carbonitrides. J Alloys Compd 265:215–233. https://doi.org/10.1016/S0925-8388(97)00297-1
Lengauer W, Ettmayer P (1990) Recent advances in the field of transition-metal refractory nitrides. High Temp High Press 22:13–24
Sun Y, Bell T (1997) A numerical model of plasma nitriding of low alloy steels. Mater Sci Eng A 224:33–47. https://doi.org/https://doi.org/10.1016/S0921-5093(96)10561-X
Hägg G (1928) X-ray studies on the ‘nitrides’ of iron. Nature 121:826–827. https://doi.org/10.1038/121826a0
Rognerud EG, Rom CL, Todd PK et al (2019) Kinetically controlled low-temperature solid-state metathesis of manganese nitride Mn3N2. Chem Mater 31:7248–7254. https://doi.org/10.1021/acs.chemmater.9b01565
Glück T (1994) Mechanisms of nitriding electrolytic manganese metal. Chem Eng J Biochem Eng J 54:167–173. https://doi.org/10.1016/0923-0467(94)00206-1
Buc MM (2016) Convenient synthesis of nanocrystalline powders of phase-pure manganese nitride g-Mn 3 N 2. 8177–8186. https://doi.org/https://doi.org/10.1007/s10853-016-0094-2
Niewa R (2002) Nitridocompounds of manganese: manganese nitrides and nitridomanganates. Zeitschrift fur Krist 217:8–23. https://doi.org/10.1524/zkri.217.1.8.20801
Yang R, Haider MB, Yang H et al (2005) Scanning tunneling microscopy study of the structural phase transformation in manganese nitride: θ-MnN → η-Mn 3 N 2. Appl Phys A Mater Sci Process 81:695–700. https://doi.org/10.1007/s00339-005-3230-4
Leineweber A, Niewa R, Jacobs H, Kockelmann W (2000) The manganese nitrides η-Mn3N2 and θ-Mn6N(5 + x): nuclear and magnetic structures. J Mater Chem 10:2827–2834. https://doi.org/10.1039/b006969h
Song X, Sun Z, Huang Q et al (2011) Adjustable zero thermal expansion in antiperovskite manganese nitride. Adv Mater 23:4690–4694. https://doi.org/10.1002/adma.201102552
García J, Bartolomé J, González D et al (1983) Thermophysical properties of the intermetallic Mn3MN perovskites I. Heat capacity of the manganese nitride Mn4N. J Chem Thermodyn 15:465–473. https://doi.org/10.1016/0021-9614(83)90044-7
Michalsky R, Pfromm P (2019) Thermochemical ammonia and hydrocarbons, U.S. Patent No. 10,315,967. U.S. Patent and Trademark Office, Washington, DC
Shan N, Chikan V, Pfromm P, Liu B (2018) Fe and Ni Dopants Facilitating Ammonia Synthesis on Mn4N and mechanistic insights from first-principles methods. J Phys Chem C 122:6109–6116. https://doi.org/10.1021/acs.jpcc.7b12569
Heidlage MG, Kezar EA, Snow KC, Pfromm PH (2017) Thermochemical synthesis of ammonia and syngas from natural gas at atmospheric pressure. Ind Eng Chem Res 56:14014–14024. https://doi.org/10.1021/acs.iecr.7b03173
Selg H, Meka SR, Kachel M et al (2013) Nitriding behaviour of maraging steel: experiments and modelling. J Mater Sci 48:4321–4335. https://doi.org/10.1007/s10853-013-7248-2
Verdiere A, Hofer C, De Waele S et al (2017) Precipitation in simultaneously nitrided and aged Mo-containing maraging steel. Mater Charact 131:21–30. https://doi.org/10.1016/j.matchar.2017.06.014
Mittemeijer EJ, Somers MA (2015) Thermochemical surface engineering of steels. Woodhead Publishing
Acknowledgements
This research was supported by the National Science Foundation under Grant No. 1856084 for the FEWtures project. We gratefully acknowledge access to the Franceschi Microscopy and Imaging Center, L.J. Smith Hall, at Washington State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi Aframehr, W., Pfromm, P.H. Activating dinitrogen for chemical looping ammonia synthesis: nitridation of manganese. J Mater Sci 56, 12584–12595 (2021). https://doi.org/10.1007/s10853-021-06079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06079-7