Abstract
A systematic investigation of the electrochemical iron nitride synthesis in a LiCl/KCl salt melt at 723 K shows an optimum of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) formation in the range of 2.2 to 2.3 V and for \(\gamma '\)-Fe\(_4\)N between 2.4 and 2.5 V enabling the control of the desired iron nitride phase by setting the supplied terminal voltage. The product formation of iron nitrides starts when the electrochemical window is reached, which could be verified by linear sweep voltammetry. Hence, a maximum of nitrogen content in the formed iron nitride phases is observed for 2.27 V. During elongated synthesis periods, convection emerges as the predominant transport mechanism hindering an accelerated reaction rate with higher overpotential applied. Real-time analysis of the background current allows conclusions about the remaining nitride concentration. Additionally, there is concurrent iron nitride formation at the electrode surface through nitride adsorption and autonucleation-induced precipitation of iron and nitride ions. The analysis of the amount of sediment in comparison to the layer thickness of the nitrided working electrode suggests that the autonucleation mechanism dominates over the adsorption mechanism with increasing overpotential and can be further enhanced by this feature.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron nitrides have entered various fields as important materials for many decades [1]. For example, the surface hardening of iron and steel by nitridation describes an important process in steel treatment. Particulary, the nitrides \(\gamma '\)-Fe\(_4\)N and \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) are formed as surface coatings for corrosion protection [2]. While the embedding of small amounts of nitrogen into the iron lattice of low-alloy steels will lead to an increased sensitivity to aging and blue brittleness, in austenitic steels, it can greatly improve mechanical properties such as strength, especially in high-temperature applications [3]. In addition, \(\gamma '\)-Fe\(_4\)N as bulk material was used as magnetic data storage media [4, 5]. The iron-nitrogen phase diagram shows different phases up to a nitrogen content of approx. 33 mol% (cf. Fig. 1). Further, metastable phases \(\alpha '\)-Fe\(_8\)N [6] and \(\alpha ''\)-Fe\(_{16}\)N\(_2\) [6, 7] were investigated as novel rare earth metal-free magnetic materials. \(\alpha ''\)-Fe\(_{16}\)N\(_2\) nanoparticles show enhanced saturation magnetization at high fields compared to commercial \(\gamma\)-Fe\(_2\)O\(_3\) nanoparticles [8]. Metastable FeN\(_{1+x}\) was synthesized in wurtzite [9] and rocksalt-type structures [10], apparently depending on the nitrogen content [11, 12]. After theoretical prediction [13], a high-pressure nickel arsenide type FeN modification was found by Clark et al. [14]. At even higher pressures, FeN\(_2\) and FeN\(_4\) form from the elements upon laser heating [15]. However, in addition to the \(\alpha\)-Fe substrate, the phases \(\gamma '\)-Fe\(_4\)N and \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) are the focus of this work.
Phase diagram of the system iron-nitrogen. Reprinted with permission from [16]. Copyright 2008 American Chemical Society
Nitridation of steels is often achieved using ammonia via gas or plasma nitridation techniques [17,18,19]. Further, preparation methods of iron nitrides include chemical vapor deposition [20] or reactive ion beam sputtering [21], but also metathesis reactions using ammonia either at standard pressures [22] or in its supercritical state [23] are possible. An overview of various methods is given in [1]. Most of these processes use ammonia, especially in industrial applications. This poses a significant problem in achieving the climate targets in accordance with the Paris Climate Agreement due to the energy-intensive production of ammonia [24]. With 1.8% of global energy output and 500 million tonnes of CO\(_2\) annually (as of 2020), ammonia production is one of the “big four” industrial processes alongside cement, steel, and ethane production [25].
One option for nitridation avoiding the use of ammonia is the electrochemical surface nitriding in molten salts. Within this method, molecular nitrogen can be used as the nitride source instead of hazardous ammonia. Furthermore, in conventional gas nitriding, higher temperatures ranging from 770 to 870 K are required. Phase control is attained by adjusting the nitriding potential of ammonia, which is facilitated through temperature regulation [17]. In electrochemical surface nitridation, however, lower temperatures of 723 K are sufficient, and with the voltage as a control parameter, there is also a simple option for nitrogen activation. Electrochemical surface nitriding was first achieved by Goto back in 1994 for titanium nitride [26] using Li\(_3\)N, KNO\(_3\), or KNO\(_2\) as nitride sources, dependent on the electrode polarization, but it has remained less prominent compared to conventional methods of preparation. This is primarily due to its inherent inaccessibility, originating from the high sensibility to air moisture or foreign ions of molten salt systems, resulting in limited process control even at the laboratory scale. The electrochemical nitridation of iron has been reported as well, although the data situation here is scarce. In a proof of concept study including thermodynamic considerations, it was shown that \(\gamma '\)-Fe\(_4\)N can be produced in LiCl/KCl molten salt [27], but this iron nitride synthesis could not be confirmed later by Monson and Pearce [28]. Ito modified the process by use of a nitrogen gas electrode as nitride source [29]. Later, Lehmann used this method to successfully synthesize both the nitrogen-poor phase \(\gamma\)-FeN\(_{0.134}\) and the nitrogen-rich phase \(\epsilon\)-Fe\(_3\)N\(_{1.51}\) [30]. In addition, a correlation between synthesis time and layer thickness of the nitrided material was observed [27, 30], implying that Faraday’s law is also valid in molten salts. However, the progression of chronoamperometric experiments in iron nitride synthesis was not discussed yet.
Precise knowledge of the actual voltage present at the working electrode requires the use of a three-electrode system with a reference electrode (RE). Typically, a Ag/AgCl RE is used [31, 32], whose equilibrium potential fluctuates above 500 mV depending on the AgCl concentration [33]. Furthermore, the used Pyrex membrane [34] often does not allow repeated use in a LiCl/KCl salt melt due to corrosion. This drawback results from small impurities of water and hydroxide anions especially in hygroscopic LiCl [35] Beside from that, the eutectic mixture of LiCl/KCl provides an electrochemically stable electrolyte with a high solubility for Li\(_3\)N, a large electrochemical window of 3.6 V at the common working temperature of 723 K [36] and low costs. In order to make electrochemical nitridation more attractive for nitride synthesis and particularly achieve a more sustainable iron nitride production process, this work investigates the extent to which it is possible to ensure product control even in a two-electrode setup without a RE. Additionally, a detailed interpretation of the current-time curves provides the possibility of real-time monitoring of the electrochemical nitridation of iron.
Theoretical background
The electrochemical cell used for the nitridation of iron comprises two electrodes. While a cathodically polarized iron foil serves as working electrode, an anodically polarized nitrogen gas electrode made from molybdenum is used as counter electrode. The gas electrode is constructed analogously to a standard hydrogen electrode, and the reduction of nitrogen gas takes place at the contact surface between the electron conductor, electrolyte, and nitrogen gas via
The nitride ions formed in this way are subsequently transported from the counter to the working electrode. In a chronoamperometric (CA) experiment, a constant voltage is applied between these two electrodes and the current flow is recorded over time. Nitride ions are transported within a molten salt system via two different mechanisms. In the first couple of seconds, diffusion is expected to be the dominant process [37]. For purely diffusion-controlled processes, the Faraday current flow I(t) can be described by the Cottrell equation [38]:
where \(n=3\) is the number of electrons transferred, F is the Faraday constant, A is the electrode surface, D is the diffusion coefficient, and c describes the average concentration of the diffusing species. After these first seconds, natural convection becomes prominent, i.e., movement of particles due to density gradients. These density gradients can originate due to thermal or gravitational differences, where thermal convection is not considered here, as a constant temperature is assumed throughout based on the control of the tube furnace. Convection is the dominant transport mechanism in long synthesis times of several minutes [37] and is noticeable by a constant residual current flow, which is controlled by means of
It is characterized by the thickness \(\delta _{\text {conv}}\) of the convection-diffusion layer. The transition from purely diffusion-controlled mass transport to convection-controlled transport is described by the build-up of the diffusion layer \(\delta _{\text {diff}}\), which grows with time and reaches \(\delta _{\text {conv}}\) at its limit. Accordingly, a current curve is expected, which initially drops steeply in a purely diffusion-controlled manner (cf. Eq. 2) and subsequently manifests itself at a constant level (cf. Eq. 3). Since the gas flow with 10–30 sccm is low, perturbation of the system is only regarded as a limited factor.
The movement of nitride ions in molten salt is a possible piece of the puzzle on the way to a microscopic description of the formation of metal nitrides in molten salt. According to current knowledge, two possible reaction mechanisms compete for the formation of metal nitrides. On the one hand, the nitride ion can penetrate the Stern double layer and form adsorbed atomic nitrogen at the working electrode via oxidation [27] according to
The nitrogen adsorbed in this way subsequently incorporates into the structure of the working electrode metal and forms the corresponding metal nitride phase on the surface of the electrode, iron nitride in the present case. Furthermore, in the absence of nitride ions in the molten salt, it was reported that the working electrode is anodically oxidized and degraded [27]. This leads to the autonucleation mechanism reported [28] as a second possibility in which the iron in the working electrode is oxidized and dissolved into the salt melt. Both metal cations and nitride anions will subsequently meet within the salt melt and precipitate from the solution. Accordingly, the location of the product nitride phase would indicate the mechanism of formation. As an undesired side reaction, the recombination of two adsorbed nitrogen atoms to nitrogen gas at the working electrode must be taken into account. For this reaction, no product formation can be observed despite additional current flow, which should be considered when monitoring the CA experiment and prevented if possible.
Methods
The syntheses of the iron nitrides took place under inert gas atmosphere in a two-electrode apparatus modified from [30] but made of borosilicate glass (DWK Life Sciences, cf. Fig. 2). LiCl (99%, Grüssing) and KCl (purest. Riedel-de Haën) were dried first under dynamic vacuum at 220 \(^\circ\)C for 72 h to remove attached air moisture. Elemental analysis via hot gas extraction (ONH836, Leco) revealed that the working electrode had an oxygen, nitrogen, and hydrogen content corresponding to an overall composition of FeO\(_{0.00014(8)}\)N\(_{0.00009(3)}\)H\(_{0.0002(2)}\), while the contents of LiCl after drying corresponded to LiClO\(_{0.0088(2)}\)H\(_{0.0118(4)}\) and of KCl to KClO\(_{0.00141(2)}\)H\(_{0.0039(2)}\), respectively. In a glove box (MBraun), 400 mmol of an eutectic mixture of LiCl and KCl (59:41 mol%) were mixed with 2 mmol of Li\(_3\)N and transferred into a SiC crucible (QSIL Ingenieurkeramik GmbH). The preparation of the Li\(_3\)N was carried out according to [39]. The filled crucible was placed inside a glass tube (cf. [30]), transferred outside the glove box into a vertical tube furnace, and brought to the working temperature of 450 \(^\circ\)C in a dynamic argon atmosphere. The nitrogen gas counter electrode consists of a borosilicate glass tube immersed in the molten salt and a molybdenum wire (99.97%, Plansee) as an electron conductor, which was bent several times to increase the surface area. The working electrode is a freshly polished iron foil (99.8%, Goodfellow) with an electrode surface of ca. 3 cm² (measured by immersion of the electrode), which was suspended by another molybdenum wire. After synthesis, the working electrode was transferred under standard Schlenk techniques into freshly degassed water to remove any adhering salt. The SiC crucible was transferred to another Schlenk bottle filled with degassed water to remove all of the electrolytes. The sediment was then filtered and dried under a dynamic vacuum.
Sample head made of borosilicate glass for electrochemical nitride synthesis in the two-electrode setup. Nitrogen gas reaches the counter electrode (spiral-shaped wire, right) via a gas supply. The working electrode consists of an iron foil (lower right). The apparatus is sealed airtight via a glass body (not shown) and two septa. The upper right shows the SiC crucible (80 mm height)
Electrochemical nitriding was recorded with a combination of a source measurement unit (SMU, Toellner 8852) and data logger (Agilent 34970A with 34902A data aquisition (DAQ) card) in the early experiments, then later with the help of a SP-300 galvanostat/potentiostat (Biologic). X-ray powder diffraction patterns were recorded with a Stadi-P powder diffractometer (Stoe & Cie GmbH) with Mo-K\(\alpha\) radiation (\(\lambda =70.93\) pm). The determination of the lattice parameters from the powder diffractometry data was carried out with the programs of FullProf Suite, version January 2021 [40]. Scanning electron microscopy (SEM) was performed on a GeminiSEM 560 by Zeiss. Profilometry has been carried out with a ContourX-500 by Bruker. Atomic force microscopy (AFM) pictures were taken using a Dimension 3100 device (Veeco, now Bruker) together with an NCHR tip (NanoWorld) in tapping mode.
Results and discussion
A significant challenge in the electrochemical nitridation in molten salt is to directly observe the system during synthesis. Currently, it is not possible to determine, for instance, whether the electrode arrangement is slightly unstable due to the gas flow, whether excessive corrosion occurs at the glass insert, or if inhomogeneous growth poses a risk of short-circuiting as the working and counter electrodes come into contact. At present, the only feasible possibility of monitoring is the response of the electrochemical cell to the applied terminal voltage. The interpretation of these results enabled a targeted optimization of the experimental setup, leading to a reproducible nitridation of the working electrode. In this context, 20 iron foils serving as working electrodes were exposed in chronoamperometric experiments at different terminal voltages. After each experiment, the working electrode was coated with a black layer and/or there was a black precipitate within the salt electrolyte in the reaction crucible. As far as possible, both the working electrode and the precipitate were recovered and analyzed using powder X-ray diffraction (XRD). However, due to the varying extent of degradation of the working electrode, this was not always possible. For this reason, the focus in this work was put on the variation of the terminal voltage, and the effects on product formation were investigated. A short overview of different product phases found in the different runs is given in Table 1, while a more detailed collection can be found in the Supplement.
Characteristics of the chronoamperometric experiments
Whenever iron nitride formation is observed after a CA experiment, it manifested itself by a deviation from the description of Eq. 2. Cottrellian behavior can be obtained by a linear component at the beginning of the measurement in transforming the abscissa via \(t\rightarrow t^{-0.5}\). The first 5 s are excluded from this consideration as they reflect mainly capacitive currents. As an example, sample 16 is used to explain the interpretation of the CA experiments and to derive relevant parameters. Data from further experiments within this series can be found in the Supplementary Material.
Chronocoulometric analysis of sample 16. Top: Current trace plotted against \(t^{-0.5}\). The second linear region describes the diffusion-driven mass transfer at the beginning of the experiment and marks the lower boundary for the subsequent integration based on the offset to the regression line. Bottom: Time-dependent current curve of the synthesis of \(\gamma '\)-Fe\(_4\)N with curve fitting for the diffusion-controlled initial range (more detailed in inset) and subsequent linear extrapolation of the background current (green area). The additional fluxed charge (gray area) is attributed to the iron nitride formation. Data for the other experiments are given in the Supplement, Chapter 1
The offset in Fig. 3 (top) represents the first possible point at which product formation begins and represents the dominant component. In the course of the subsequent synthesis (cf. Fig. 3 (bottom)), the background current overlaps with the substance conversion, which provides an additional current flow. The background current is considered purely convective from the offset after 21 s and behaves time-independent according to Eq. 3. The transition between diffusion- and convection-dominated mass transfer after 21 s is in agreement with [37], who detected a constant current flow after approx. 10 s. The additional synthesis-induced current flow increases strongly before it decreases with an almost constant slope. The initial current increase is due to the oxidation of the nitride on the iron surface according to Eq. 4. The subsequent reaction of the adsorbed atomic nitrogen with the iron leads to the formation of metallic \(\gamma '\)-Fe\(_4\)N on the working electrode, which results in a voltage drop. This layer reacts quickly at first due to the large surface area, but the reaction rate constantly decreases because the nitrogen atoms have to migrate increasingly deeper into the iron foil. The formation of \(\gamma '\)-Fe\(_4\)N layer on the surface is accompanied by an increase in the specific resistance \(\rho\), rising from approximately \(0.1~\mu \Omega ~\text {m}\) for pure iron [41] to \(162~\mu \Omega ~\text {m}\) for \(\gamma '\)-Fe\(_4\)N [42] at room temperature. The combination of the increasing resistance of the working electrode and the decreasing reaction rate of Eq. 4 leads to a steady decrease of the net current. For better comparison, the synthesis time was fixed to 150 min. Larger fluctuations in current may result from detachment of a part of the formed iron nitride from the thus refreshed surface.
The level of the constant background current (cf. Fig. 3 (bottom, green)) is approximated by a straight line that runs on the equal height of the current profile of the last few minutes. Given this purely convective current flow \(I_{\text {conv}}\), Eq. 3 enables the possibility to estimate the remaining nitride concentration in solution and therefore an approximation of the synthesis progress as shown in Fig. 4.
Estimated range of the remaining nitride concentration \(c(\text {N}^{3-})\) as a function of the background current \(I_{\text {conv}}\) according to Eq. 3 (purple area). The limits shown follow from the range of diffusion-convection layer thickness \(\delta _{\text {conv}}\) of 128–163 µm (blue line and green line resp.) [37]. The markers symbolize values of \(I_{\text {conv}}\) for various experiments. Data can be found in the Supplement. The red line marks the initial concentration of Li\(_3\)N as the starting electrolyte
Depending on the actual convection-diffusion layer, a background current of 150±20 mA results for the initial amount of Li\(_3\)N added. In most of the experiments, a background current was observed that resulted in a smaller amount of Li\(_3\)N than was initially added. This means that less Li\(_3\)N is present in the electrolyte at equilibrium. The reason lies in the low reaction rate of the nitrogen reduction at the counter electrode, as the ionization currents in gases are generally very low. Furthermore, the electrolyte cannot be completely dried from moisture residues, as shown by Nikolaev et al. [35]. Accordingly, the oxidation of hydroxide to molecular oxygen can occur as a competing reaction to nitride oxidation. Based on elemental analysis via hot gas extraction measurements, there was a residual oxygen content of clearly less than 1% in the electrolyte before synthesis, which would correspond to a background current of around 100 mA if the reaction was completed within 150 min. However, it can be assumed that the melting of the electrolyte and the constant permeation of the electrolyte with nitrogen gas expel a large proportion of the residual moisture, which explains the significantly lower background currents in most experiments. The remaining net current (cf. Fig. 3 (bottom, gray)) represents the substance conversion assuming that nitride anions are the only component being oxidized. Integration of the current over time yields the charge quantity Q in the amount of
As given in the correlation of layer thickness on the reaction time [27, 30], Faraday’s law can be applied in order to connect the charge with the amount of substance:
It is noteworthy that the amount of substance calculated by Eqs. 6 and 5 represents a maximum amount of substance. Since in a two-electrode setup, both the anode and cathode resistances may change over time, only a part of the excess charge is used for iron nitride formation.
Phase analysis of the nitridated iron foils
The assumptions made in the previous paragraph allow a rough quantitative analysis of the nitridation process. In order to not only monitor the amount of nitride ions being incorporated into the iron lattice, but also control the formation of a specific iron nitride phase, a qualitative analysis of the resulting product composition is required. In this context, both the surface of the working electrode and the black precipitate recovered from the salt melt at the bottom of the crucible were investigated if possible via XRD. For instance, in sample 14, the chronocoulometric analysis of which has been carried out in "Characteristics of the chronoamperometric experiments" section, only traces of precipitate were found after the aqueous work-up, which is why only the working electrode could be analyzed using XRD. In Fig. 5, the Le Bail profile fit of sample 14 shows evidence of the formation of exclusively \(\gamma '\)-Fe\(_4\)N at the surface.
Le Bail profile fit of the XRD pattern obtained from the surface of the working electrode of sample 14 (measured data in red, fit profile in black, difference plot in blue and Bragg positions for \(\alpha\)-Fe [43] and \(\gamma '\)-Fe\(_4\)N [44] in green). Top right: Surface of the corresponding working electrode
From the profile fit of the obtained XRD pattern, the lattice parameter for \(\gamma '\)-Fe\(_4\)N can be extracted. Within the narrow homogeneity range of \(\gamma '\)-Fe\(_4\)N, a linear dependence of the lattice parameter a with the nitrogen content was derived earlier [45]:
where \(c_N\) is the nitrogen content in atomic %. An analog consideration provides the dependence of the lattice parameters a and c for \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) on the nitrogen content in \(\epsilon\)-FeN\(_y\) (\(y=(1+x)/3\)) for quenched material [46]:
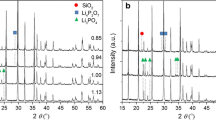
Sample 11 (cf. Supplement) shows the generation of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) and \(\gamma '\)-Fe\(_4\)N in parallel and that a separation of the reflections is quite possible. For the sample shown in Fig. 5 with a cubic lattice parameter a = 377.85(4) pm, this results in a chemical composition of Fe\(_4\)N\(_{0.916(3)}\). Lattice parameters and the resulting composition of the desired iron nitride phases are listed in the Supplement, paragraph 3, for all samples. The same type of analysis can be carried out for the precipitate. As no precipitate could be analyzed for sample 16, Fig. 6 shows the precipitate of sample 17 after the electrolyte is washed out with water. Sample 17 was performed virtually at the same voltage as used for sample 16.
Like sample 16, this precipitate consists mainly of \(\alpha\)-Fe and \(\gamma '\)-Fe\(_4\)N. Analysis of the \(\gamma '\)-Fe\(_4\)N phase reveals a composition of \(\gamma '\)-Fe\(_4\)N\(_{1.00(5)}\). Finding large amounts of \(\alpha\)-Fe at the bottom of the crucible alongside the intended iron nitride phases enables a third transport mechanism governing electrochemical processes within this electrolysis cell. If there is an insufficient amount of nitride ions present in solution, the iron cations will be transported towards the counter electrode, undergo discharge, and subsequently precipitate out of the solution. The third phase being identified is Fe\(_3\)O\(_4\). This is likely formed due to the washing process; if not, degassed water is applied (early experiments). With regard to the formation of iron oxides, degassing the water has proven to be an effective measure. XRD patterns taken on several working electrodes from different experiments confirmed the formation of \(\gamma '\)-Fe\(_4\)N as well as \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) (cf. Table 1). In a single case, the formation of \(\gamma\)-Mo\(_2\)N was observed in the precipitate of sample 08 stemming from the molybdenum wire to which the working electrode is attached. In this single case, this molybdenum wire probably was partly immersed in the electrolyte.
Effects of the applied terminal voltage on the composition of the target product
According to the scheme shown above, CA experiments were carried out with respect to substance conversion and iron nitride formation as a function of the applied terminal voltage. Table 1 shows the formation of different iron nitride phases depending on the applied terminal voltage as well as the maximum amount of nitride being converted according to chronocoulometric analysis. For a better overview, the salts LiCl and KCl were left out of this table, although they were still partly visible in the corresponding powder patterns after rinsing with degassed water. Furthermore, partial degradation of the corresponding iron nitrides to iron oxides during the aqueous work-up took place resulting in iron oxide impurities. After purification, the remaining iron nitride product will decompose slowly to iron oxides if stored in air, particularly if formed with small particle sizes. Electrochemically produced iron nitrides should therefore be stored under inert conditions. In addition, the formation of metallic beads was often observed at the top of the electrolyte, especially after the application of high voltages as of 2.5 V. They dissolve in water under gas formation and strongly increase the pH value. Those beads likely consist of elemental lithium, formed by the reduction of lithium ions at the counter electrode, as was earlier observed in similar systems [36]. Based on these data and the respective Le Bail profile fits of the corresponding powder diffractograms, the nitrogen content and the amount of substance conversion are shown as a function of the applied voltage in Fig. 7.
Plot of the nitrogen content of the resulting iron nitride phases against the applied terminal voltage during CA experiments of 150 min duration. The blue markers describe the nitrogen content of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) determined by Eq. 9. The green markers represent the nitrogen content of \(\gamma '\)-Fe\(_4\)N calculated using Eq. 7
While for short CA experiments of only 60 s a constant increase of nitrogen content in the desired iron nitride phase has been observed for higher overpotentials [27], this does not hold for longer durations of the chronoamperometric experiment. Rather, an optimum of nitrogen uptake seems to be present in the range of 2.2–2.3 V. At lower voltages, \(\gamma '\)-Fe\(_4\)N\(_{0.996(1)}\) formed at 2.1 V; below, no nitride formation was observed. This can be explained using linear sweep voltammetry (LSV). If the direct representation of the current on the applied voltage is not considered, but rather its derivative dU/dI, a plateau can be seen from about 2.2 V (see Fig. 8). This cusp coincides surprisingly accurately with the appearance of the iron nitride, but possibly also marks the beginning of lithium reduction. Accordingly, it appears to be essential for the synthesis of iron nitrides that the electrochemical window of the electrolyte is just barely tapped. At low overpotentials and thus low concentrations of elemental lithium, the nitrogen-rich \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) is formed, while at higher overpotentials, the less nitrogen-rich \(\gamma '\)-Fe\(_4\)N is formed. This finding, which at first glance appears contradictory, can be explained by the oxidation of the working electrode. As the potential rises, it increasingly corrodes, causing more iron ions to dissolve. This is shown by the increasing amount of \(\alpha\)-Fe at higher potentials.
Accordingly, two voltage ranges can be identified in which an iron nitride phase can be reproducibly formed. In the range from 2.20 to 2.30 V, \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) is produced, while from 2.30 to 2.52 V, the less nitrogen containing \(\gamma '\)-Fe\(_4\)N is formed. Within the respective potential interval for the corresponding iron nitride phase, there is no significant change in the nitrogen content in the iron nitride phase with the selected terminal voltage. This is presumably due to the different, not precisely specified ohmic voltage losses during synthesis, which have a slight influence on the actual electrochemical potential at the working electrode in the molten salt. The two experiments at 2.50 V and 2.52 V as well as those at 2.33 V and 2.36 V resulted in the formation of \(\gamma '\)-Fe\(_4\)N with a significantly increased nitrogen content. Within those samples, the nitrogen content surpassed the established homogeneity range, as illustrated in Fig. 1. This corresponds to the trend previously observed by Lehmann during electrochemical synthesis of \(\epsilon\)-Fe\(_3\)N\(_{1.51}\) [30]. In the case of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\), different regression lines were observed for the application of Vegard’s law depending on whether the samples cooled down at rest or were thermally quenched [46]. However, such a comparison does not exist for \(\gamma '\)-Fe\(_4\)N. Therefore, it can be assumed that the actual nitrogen content will be somewhat lower. Nevertheless, the products of samples 13, 14, 15, and 19, with unit cell parameters a of approximately 384 pm, show a significant increase in unit cell parameters compared to previously reported samples with values around 379 pm [44, 45]. The selected terminal voltage of 2.3 V is a special feature. Both \(\epsilon\)-Fe\(_3\)N and \(\gamma '\)-Fe\(_4\)N are observed here. Nevertheless, the frequency and distribution of both iron nitride phases occurring at this voltage indicate that this voltage corresponds to the phase boundary (cf. Fig. 1) between the \(\epsilon\)-Fe\(_3\)N and \(\gamma '\)-Fe\(_4\)N mixed phase and pure \(\gamma '\)-Fe\(_4\)N. In contrast to Fig. 7 (top) where a correlation between product control and applied terminal voltage could be established, the chronocoulometric analysis reveals no direct link of applied terminal voltage and amount of nitride ions being formed.
Mechanistic considerations of iron nitride formation
The amount of nitride ions formed appears to be independent on the applied terminal voltage. On average, a substance quantity of \(n\left( \text {N}^{3-}\right) =1.45\) mmol results for each synthesis experiment. As already mentioned, three reaction pathways are likely for the nitride ions in the electrolyte and are shown in the following scheme:
Within this scheme, the nitride ions in solution (central N\(^{3-}\)) can either react with dissolved iron ions inside the electrolyte forming iron nitride precipitate (pathway to the left) or meet the surface at the working electrode (pathway to the right). Here, they can either recombine to nitrogen gas in an undesired side reaction or diffuse into the surface forming an iron nitride layer. As proposed via Fig. 4, the reaction

reaches equilibrium at the far left side. While no nitrogen gas is inserted into the system during heat up overnight, this equilibrium is constantly shifting to the right side. After switching on the nitrogen gas flow, the partial pressure of nitrogen gas over the molten electrolyte rapidly turns to 1 bar of nitrogen gas and the equilibrium is restored.
In order to discuss how both different pathways for the nitride ion are involved in the reaction, it is assumed that the side reaction of the adsorbed nitrogen to molecular N\(_2\) is presumably present over the complete voltage range investigated, since the recombination will have a significantly lower energy barrier than the embedding of the nitrogen into the iron lattice. Having a higher overpotential applied, the concentration of adsorbed nitrogen at the surface of the WE is increased and therefore single adsorbed nitrogen atoms are no longer separated from each other by the chloride anions still present in the innermost Stern layer. Thus, the recombination is more likely to happen instead of iron nitride formation.
The two remaining pathways describe iron nitride synthesis mechanisms. The first one states that nitride ions are oxidized to atomic adsorbed nitrogen (cf. Eq. 4) [27]. Surface nitriding was not found by Monson and Pearce [28], but they observed a generation of a precipitate leading to an autonucleation mechanism, where both iron and nitride ions recombine within the electrolyte to an iron nitride phase. In the present study, we found iron nitrides at the surface of the working electrode as well as at the bottom of the crucible. However, chronocoulometric analysis can be used to determine which of the two mechanisms dominates and to what extent. For sample 16 shown in Fig. 3, a mass of 63.8 mg of \(\gamma '\)-Fe\(_4\)N\(_{0.916(3)}\) can be estimated based on the calculated amount of nitride ions being oxidized at the working electrode (cf. Eq. 6), assuming complete phase conversion as no side products were found (cf. Fig. 5). In the case of pure surface nitriding, this would lead to layer thickness of \(\gamma '\)-Fe\(_4\)N of
using a sample area A of 3 cm\(^2\) and a density \(\rho\) of 7.242 g cm\(^{-3}\) [44]. The actual layer thickness was determined using scanning electron microscopy (SEM) and profilometry (Fig. 9).
The results of both methods are consistent and reveal a layer thickness of about 5 µm which is barely 17% of the theoretical maximum layer thickness \(d_{\text {max}}\) calculated in Eq. 12. This discrepancy allows two conclusions to be drawn. Firstly, the assumption that the dissolved nitride ions are quantitatively converted to the corresponding iron nitride may not be correct. Part of the nitride is oxidized to nitrogen gas and thus eludes the actual layer thickness. On the other hand, this observation may put forward the autonucleation mechanism [28] to be the dominant formation mechanism, which explains the formation of an iron nitride-containing precipitate in competition with the incorporation of the adsorbed nitrogen into the iron lattice of the working electrode. While nitride oxidation to molecular nitrogen cannot be detected in our experiments, because the working and counter electrodes do not have separate sample chambers, a varying amount of black precipitate is found regularly in the crucible after aqueous work-up, especially for higher overpotentials. This observation in combination with the theoretically expected amount of iron nitride and the detected surface coating of the iron foil allows the conclusion that both reaction mechanisms for iron nitride formation compete.
Furthermore, the influence of the overpotential on the involvement of both reaction mechanisms must also be taken into account. The higher the applied overpotential, the more elemental \(\alpha\)-Fe was found as a precipitate. This was referred to an oxidation of the iron working electrode and the resulting reduction at the counter electrode (cf. "Phase analysis of the nitridated iron foils" section). As the overpotential increases, the concentration of iron cations in the solution rises more rapidly than that of the nitride anions, and the surplus cannot be intercepted. As the overpotential increases, less nitride ions arrive at the working electrode and can react with the surface. The dominating reaction mechanism therefore depends on the applied overpotential in such a way that the autonucleation mechanism becomes increasingly dominant with increasing overpotential. Since in this context—in addition to minor impurities with iron oxides—there is always an unknown quantity of elemental iron together with the iron nitride product in the precipitate, a gravimetric analysis is not possible. Therefore, this method cannot be used to draw any quantitative conclusions about how the autonucleation mechanism correlates with the applied overpotential. At terminal voltages of 2.5 V and higher, however, lithium reduction occurs and this circumstance drastically changes the current flow (see Supplemental Information). This results in very high currents of several amperes, and a chronocoulometric analysis is no longer appropriate. It is therefore not possible to completely suppress surface nitriding by changing the overpotential. Apart from a few individual events, the formation of iron nitrides takes place from 2.2 V and can be additionally accelerated up to an overpotential of approx. 300 mV, before the oxidative degradation of the working electrode is too high, and lithium reduction begins. This will lead to an accumulation of lithium metal in the crucible. Finally, in addition to possible recombination to nitrogen at the surface, it is also possible that the nitride ions do not reach the working electrode within the synthesis time of 150 min. In this case, either the convection must be very slow, which is according to Fig. 3 and [37] the dominant transport mechanism after a few seconds to minutes, or the diffusion at the Stern double layer is strongly hindered. In the molten salt, there develops a layer of chloride ions around the positively polarized working electrode, which must be penetrated by the nitride ion. This chloride layer becomes thicker with larger overpotential applied. Thus, the transport of the nitride anions is additionally hindered, further decreasing the reaction rate. According to the Buttler-Volmer equation [47] for large overpotentials, the resulting excess current strives for a limit since mass transport is hindered by the maximum velocity the nitride ions can migrate through the electrolyte. This may be observed here, as there is no increasing amount of iron nitride material to be found with higher overpotentials. However, the synthesis time remained as a parameter and not as a variable in order to better ensure comparability. Both effects will therefore even out to a certain level and give no obvious correlation in Fig. 7 at first glance.
Conclusion
The electrochemical synthesis of the iron nitrides \(\gamma '\)-Fe\(_4\)N and \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) in LiCl/KCl salt melt using chronoamperometry was performed depending on the applied terminal voltage. The current-time curves exhibit a strong deviation from purely diffusive or convective behavior whenever increased iron nitride formation occurres. An analysis of the excess charge as a function of the applied terminal voltage shows no distinct effect on the reaction rate, which is attributed to a limitation of mass transport. The origin of the background current lies on the one hand in thermal convection, as the vertical tube furnace is open at the top to the gas inlets, and on the other hand in the oxidation of the nitride. It was found that in most experiments, only a low background current of a few mA was detected, which suggests that the automatic degassing of the electrolyte solution by nitrogen gas almost completely drives out residual moisture. The optimum voltage range for the formation of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) is located between 2.2 and 2.3 V, while \(\gamma '\)-Fe\(_4\)N is mainly formed in the range from 2.4 to 2.5 V. A LSV measurement at the cusp of the electrochemical window can help to choose the potential to be applied for the synthesis of either \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) or \(\gamma '\)-Fe\(_4\)N. Immediately after the cusp in the function from dU/dI to U, which marks the beginning of the lithium reduction, the formation of \(\epsilon\)-Fe\(_3\)N\(_{1+x}\) occurs. With a larger overpotential of more than 100 mV with respect to this cusp applied, the synthesis of \(\gamma '\)-Fe\(_4\)N is sentenced. The increasing formation of a precipitate with increasing applied terminal voltage also implies that the autonucleation mechanism dominates at higher overpotentials, while surface nitridation of the working electrode is more likely to occur at lower voltages.
The use of a AgCl/Ag reference electrode was omitted because our membranes suffer from corrosion in long-term experiments. An alternative pseudo-RE, employing an immersed Ag wire directly into the electrolyte was dismissed as well since its potential did not remain stable over time. Despite the limitations of a two-electrode setup in liquid salt experiments for electrochemical nitride synthesis, it is possible to draw qualitative analogies, since the difference in terminal voltage to the actual potential difference at the electrochemical double layer is comparatively small due to the high electrolyte concentration. However, reliable transfer to other series of experiments is only possible to a limited extent, as different cell constants or nitrogen gas flows influence the voltage drop across the electrochemical double layer. If, for technical or other reasons, no reference electrode is available, the limitation of the overpotential and the behavior of the current-time curves shown provide several anchor points that enable controlled nitridation of iron without the use of ammonia in molten salt.
References
Bhattacharyya S (2015) Iron nitride family at reduced dimensions: a review of their synthesis protocols and structural and magnetic properties. J Phys Chem C 119:1601–1622
Prenosil B, Holub J, Koutnik M (1973) Ermüdungserscheinungen im Gefüge dynamisch beanspruchter, bei 620 °C gascarbonitrierter Schichten. HTM J Heat Treatm Mat 28:89–99
Reinert U (2015) Legierungselemente - Einfluss der Legierungen auf Stahl. https://web.archive.org. Accessed 01 Feb 2024
Kim TK, Takahashi M (1972) New magnetic material having ultrahigh magnetic moment. Appl Phys Lett 20:492–494
Andriamandroso D, Fefilatiev L, Demazeau G, Fournès L, Pouchard M (1984) Mössbauer resonance studies on Sn substituted Fe4N. Mater Res Bull 19:1187–1194
Jack KH (1951) The occurrence and the crystal structure of α-iron nitride; a new type of interstitial alloy formed during the tempering of nitrogen-martensite. Proc Math Phys Eng Sci 208:216–224
Widenmeyer M, Shlyk L, Senyshyn A, Mönig R, Niewa R (2015) Structural and magnetic characterization of single-phase sponge-like bulk α″-Fe16N2. Z Anorg Allg Chem 641:348–354
Dirba I, Chandra CK, Ablets Y, Kohout J, Kmječ T, Kaman O, Gutfleisch O (2023) Evaluation of Fe-nitrides, -borides and -carbides for enhanced magnetic fluid hyperthermia with experimental study of α″-Fe16N2 and ε-Fe3N nanoparticles. J Phys D Appl Phys 56:025001
Suzuki K, Morita H, Kaneko T, Yoshida H, Fujimori H (1993) Crystal structure and magnetic properties of the compound FeN. J Alloys Compd 201:11–16
Oueldennaoua A, Bauer-Grosse E, Foos M, Frantz C (1985) Formation d’un nitrure metastable FeN de structure NaCl par pulverisation cathodique. Scr Mater 19:1503–1506
Eck B, Dronskowski R, Takahashi M, Kikkawa S (1999) Theoretical calculations on the structures, electronic and magnetic properties of binary 3D transition metal nitrides. J Mater Chem 9:1527–1537
Filippetti A, Pickett WE (1999) Prediction of a ferromagnetic ground state for NaCl-type FeN. Phys Rev B 59:8397–8400
Kartsev A, Bondorenko N (2015) Thermodynamic properties of NiAs-FeN phase from first principles. International Conference on Simulation. https://arxiv.org/pdf/1807.09900.pdf. Accessed 01 Feb 2024
Clark WP, Steinberg S, Dronskowski R, McCammon C, Kupenko I, Bykov M, Dubrovinsky L, Akselrud LG, Schwarz U, Niewa R (2017) High-pressure NiAs-type modification of FeN. Angew Chem Int Ed 56:7302–7306
Bykov M, Bykova E, Aprilis G, Glazyrin K, Koemets E, Chuvashova I, Kupenko I, McCammon C, Mezouar M, Prakapenka V, Liermann HP, Tasnádi F, Ponomareva AV, Abrikosov IA, Dubrovinskaia N, Dubrovinsky L (2018) Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains. Nat Commun 9:2756
Niewa R, Rau D, Wosylus A, Meier K, Hanfland M, Wessel M, Dronskowski R, Dzivenko DA, Riedel R, Schwarz U (2009) High-pressure, high-temperature single-crystal growth, ab initio electronic structure calculations, and equation of state of ε-Fe3N1-x. Chem Mater 21:392–398
Babul T (2013) Gas nitriding. In: Chung Y (ed) Wang QJ. Encyclopedia of tribology Springer, US, New York, NY and Springer Reference, pp 1455–1460
Karamiş MB, İpek R (1997) An evaluation of the using possibilities of the carbonitrided simple steels instead of carburized low alloy steels (wear properties). Appl Surf Sci 119:25–33
Ashrafizadeh F (2003) Influence of plasma and gas nitriding on fatigue resistance of plain carbon (Ck45) steel. Surf Coat Technol 174–175:1196–1200
Roberson SL, Finello D, Banks AD, Davis RF (1998) Growth of Fe3N films via chemical vapor deposition of iron acetylacetonate and anhydrous ammonia. Thin Solid Films 326:47–50
Iwatsubo S, Naoe M (2002) Characteristics of Fe-N films deposited by a reactive ion beam sputtering using adsorptive and ionized nitrogen. Vacuum 66:251–256
Chen XZ, Dye JL, Eick HA, Elder SH, Tsai KL (1997) Synthesis of transition-metal nitrides from nanoscale metal particles prepared by homogeneous reduction of metal halides with an alkalide. Chem Mater 9:1172–1176
Richter T, Niewa R (2014) Chemistry of ammonothermal synthesis. Inorganics 2:29–78
UNFCCC (2018) The Paris Agreement. Paris Climate Change Conference - November 2015. https://unfccc.int/documents/184656. Accessed 28 Mar 2024
The Royal Society (2020) Ammonia: zero-carbon fertiliser, fuel and energy store. https://www.royalsociety.org/green-ammonia. Accessed 01 Feb 2024
Goto T, Tada M, Ito Y (1994) Electrochemical surface nitriding of titanium in molten salt system. Electrochim Acta 39:1107–1113
Goto T, Obata R, Ito Y (2000) Electrochemical formation of iron nitride film in a molten LiCl/KCl:Li3N system. Electrochim Acta 45:3367–3373
Monson TC, Pearce CJ (2014) Electrochemical solution growth of magnetic nitrides. https://doi.org/10.2172/1172790. Accessed 01 Feb 2024
Ito Y, Goto T (2005) Electrochemistry of nitrogen and nitrides in molten salts. J Nucl Mater 344:128–135
Lehmann TS, Niewa R (2019) Electrochemical synthesis of highly nitrogen containing γ-FeN0.13 and ε-Fe3N1.51 in a molten salt system. Eur J Inorg Chem 2019:730–734
Kim K, Kim JN, Yoon HC, Han JI (2015) Effect of electrode material on the electrochemical reduction of nitrogen in a molten LiCI-KCl-CsCl system. Int J Hydrogen Energy 40:5578–5582
Horvath D, Simpson MF (2018) Electrochemical monitoring of Ni corrosion induced by water in eutectic LiCl-KCl. J Electrochem Soc 165:C226
Shirai O, Nagai T, Uehara A, Yamana H (2008) Electrochemical properties of the Ag+∣Ag and other reference electrodes in the LiCl-KCl eutectic melts. J Alloys Compd 456:498–502
Yoon S, Kang D, Sohn S, Park J, Lee M, Choi S (2020) Reference electrode at molten salt: a comparative analysis of electroceramic membranes. J Nucl Fuel Cycle Waste Technol 18:143–155
Nikolaev AY, Mullabaev AR, Suzdaltsev AV, Kovrov VA, Kholkina AS, Shishkin VY, Zaikov YP (2022) Purification of alkali-metal chlorides by zone recrystallization for use in pyrochemical processing of spent nuclear fuel. At Energy 131:195–201
Murakami T, Nohira T, Ogata YH, Ito Y (2004) Electrochemical window of a LiCl-KCl-CsCl melt. Electrochem Solid-State Lett 8:E1
Ge J, Cai B, Zhu F, Gao Y, Wang X, Chen Q, Wang M, Jiao S (2023) Natural convection in molten salt electrochemistry. J Phys Chem B 127:8669–8680
Cottrell FG (1903) Der Reststrom bei galvanischer Polarisation, betrachtet als ein Diffusionsproblem. Z Phys Chem 42U:385–431
Lehmann TS, Niewa R (2020) Electrochemical synthesis of transition metal oxide nitrides with ε-TaN, ẟ-NbN and γ′-Mo2N structure type in a molten salt system. Z Naturforsch B 75:33–40
Rodríguez-Carvajal J (2001) An introduction to the Program FullProf 2000. https://www.ill.eu/sites/fullprof/index.html. Accessed 01 Feb 2024
Arajs S, Colvin RV (1964) Electrical resistivity of high purity iron from 300 to 1300 °K. Phys Status Solidi B 6:797–802
Chen SK, Jin S, Tiefel TH, Hsieh YF, Gyorgy EM, Johnson DW (1991) Magnetic properties and microstructure of Fe4N and (Fe,Ni)4N. J Appl Phys 70:6247–6249
Owen EA, Williams GI (1954) A low-temperature X-ray camera. J Sci Instrum 31:49–54
Jacobs H, Rechenbach D, Zachwieja U (1995) Structure determination of γ′-Fe4N and ε-Fe3N. J Alloys Compd 227:10–17
Somers MAJ, van der Pers NM, Schalkoord D, Mittemeijer EJ (1989) Dependence of the lattice parameter of γ′ iron nitride, Fe4N1–x, on nitrogen content; accuracy of the nitrogen absorption datairon nitride, Fe4N1–x, on nitrogen content; accuracy of the nitrogen absorption data. Mater Trans 20:1533–1539
Liapina T, Leineweber A, Mittemeijer EJ, Kockelmann W (2004) The lattice parameters of ε-iron nitrides: lattice strains due to a varying degree of nitrogen ordering. Acta Mater 52:173–180
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York and Weinheim
Acknowledgements
The authors gratefully thank Dr. Rainer Stöhr from the Center of Applied Quantum Technology at the University of Stuttgart for performing the SEM and profilometric measurements and Sally Eickmeier for the AFM measurement.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thiebes, Y.M., Engel, K. & Niewa, R. Chronoamperometric investigations on electrochemical synthesis of iron nitrides in molten salt system. J Solid State Electrochem 28, 3601–3613 (2024). https://doi.org/10.1007/s10008-024-05935-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-024-05935-1