Abstract
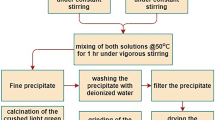
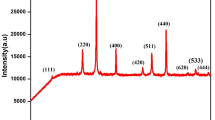
CuFeMnO4 spinel oxide was synthesized by co-precipitation method through calcination of the precipitate precursor at 500–900 °C. The reaction process was investigated by thermogravimetric analysis (TG), differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). The structure, valence state and magnetic properties of the powders obtained were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and vibrating sample magnetometer (VSM) further. The results revealed that the precipitate is composed of amorphous metal hydroxides and oxides, after which the spinel structure and several phases, such as CuO, Fe3O4 and Mn3O4, turned up in the calcined powders. As the calcining temperature raising, these phases began to transform into a single phase of CuFeMnO4. However, CuO phase segregated from the spinel structure with further calcining. The valence state of Mn ions in octahedral sites and Cu ions in tetrahedral sites were ascertained by XPS, which showed the reduction of Mn4+ and Cu+ during the calcining procedure. Besides, the magnetization of the samples increased obviously with the calcining temperature.






Similar content being viewed by others
References
Karan NS, Sarma DD, Kadam RM, Pradhan N (2010) Doping transition metal (Mn or Cu) ions in semiconductor nanocrystals. J Phys Chem Lett 1(19):2863–2866
Kharisov BI, Dias HVR, Kharissova OV (2014) Mini-review: ferrite nanoparticles in the catalysis. Arab J Chem. http://dx.doi.org/10.1016/j.arabjc.2014.10.049
Long NV, Yang Y, Teranishi T, Cao MT, Cao Y, Nogami M (2015) Synthesis and magnetism of hierarchical iron oxide particles. Mater Design 86:797–808
Quan J, Mei L, Ma Z, Huang J, Li D (2016) Cu1.5Mn1.5O4 spinel: a novel anode material for lithium-ion batteries. Rsc Adv 6(61):55786–55791
Rogov A, Mugnier Y, Bonacina L (2015) Harmonic nanoparticles: noncentrosymmetric metal oxides for nonlinear optics. J Opt 17:033001. https://doi.org/10.1088/2040-8978/17/3/033001
Hu J, Lo IM, Chen G (2005) Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 21(24):11173–11179
Long NV, Yang Y, Teranishi T, Cao MT, Cao Y, Nogami M (2015) Related magnetic properties of CoFe2O4 cobalt ferrite particles synthesised by the polyol method with NaBH4 and heat treatment: new micro and nanoscale structures. Rsc Adv 5(70):56560–56569
Randhawa BS, Dosanjh HS, Kaur M (2009) Preparation of spinel ferrites from citrate precursor route—a comparative study. Ceram Int 35(3):1045–1049
Zhang G, Qu J, Liu H, Cooper AT, Wu R (2007) CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 68(6):1058–1066
Long N, Yang Y, Yuasa M, Thi C, Cao Y, Nann T, Nogami M (2013) Controlled synthesis and characterization of iron oxide nanostructures with potential applications for gas sensors and the environment. Rsc Adv 4(4):6383–6390
Zhang R, Liu J, Wang S et al (2011) Magnetic CuFe2O4 nanoparticles as an efficient catalyst for CO cross-coupling of phenols with aryl halides. Chemcatchem 3(1):146–149
Birajdar DS, Devatwal UN, Jadhav KM (2002) X-ray, IR and bulk magnetic properties of Cu1+xMnxFe2–2xO4 ferrite system. J Mater Sci 37(7):1443–1448. https://doi.org/10.1023/A:1014505620254
Patil BL, Sawant SR, Patil SA, Patil RN (1993) The electrical properties of Ge4+ substituted copper ferrite. Czech J Phys 43(2):179–185
Patil BL, Sawant SR, Patil SA, Patil RN (1994) Electrical properties of Si4+ substituted copper ferrite. J Mater Sci 29(1):175–178. https://doi.org/10.1007/BF00356590
Patil BL, Sawant SR, Suryavanshi SS, Patil SA (1993) Conduction mechanism in Sn4+ substituted copper ferrite. B Mater Sci 16(4):267–271
Ayieko CO (2016) Optical characterization of TiO2-bound (CuFeMnO4) absorber paint for solar thermal applications. Am J Energy Res 4(1):11–15
Long NV, Yang Y, Teranishi T, Thi CM, Cao Y, Nogami M (2015) Biomedical applications of advanced multifunctional magnetic nanoparticles. J Nanosci Nanotechnol 15(12):10091
Orel ZC (1999) Characterisation of high-temperature-resistant spectrally selective paints for solar absorbers. Sol Energy Mater Sol Cells 57(3):291–301
Tanaka Y, Takeguchi T, Kikuchi R, Eguchi K (2004) Steam reforming of dimethyl ether over composite catalysts of alumina and Cu–Mn–Fe spinel oxide. In: Meeting Abstracts—205th Meeting of The Electrochemical Society, MA 2004 01, p 668
Fan Y, Siriwardane R, Tian H (2015) Trimetallic oxygen carriers CuFeMnO4, CuFeMn2O4, and CuFe0.5Mn1.5O4 for chemical looping combustion. Energy Fuel 29(10):150909010850007
Kaluža L, Šurca-Vuk A, Orel B, Dražič G, Pelicon P (2001) Structural and IR spectroscopic analysis of sol–gel processed CuFeMnO4 spinel and CuFeMnO4/silica films for solar absorbers. J Sol–Gel Sci Technol 20(1):61–83
Gingasu D, Mindru I, Patron L et al (2012) Synthesis of CuGa2O4 nanoparticles by precursor and self-propagating combustion methods. Ceram Int 38(8):6739–6751
Hernandez-Moreno MJ, Ulibarri MA, Rendon JL, Serna CJ (1985) IR characteristics of hydrotalcite-like compounds. Phys Chem Miner 12(1):34–38
Köseoğlu Y (2013) Structural, magnetic, electrical and dielectric properties of MnxNi1−xFe2O4 spinel nanoferrites prepared by PEG assisted hydrothermal method. Ceram Int 39(4):4221–4230
Sang-Koo K, Ken Apos K et al (2005) Inhibition of conversion process from Fe(OH)3 to β-FeOOH and α-Fe2O3 by the addition of silicate ions. ISIJ Int 45(1):77–81
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56(10):978–982
Paufler P (2010) R. A. Young (ed) The Rietveld method. International Union of Crystallography. Oxford University Press 1993. 298 p. Price £ 45.00. ISBN 0–19–855577–6. Cryst Res Technol 30(4):494
Lenglet M, D’Huysser A, Kasperek J, Bonnelle JP, Dūrr J (1985) Caracterisation des etats d’oxydation du cuiver et du manganese dans quelques manganites par analyse des spectres XPS, d’emission X et des seuils d’absorption X. Mater Res Bull 20(7):745–757
Bayón R, Vicente GS, Maffiotte C, Morales Á (2008) Characterization of copper–manganese–oxide thin films deposited by dip-coating. Sol Energy Mater Sol Cells 92(10):1211–1216
Gillot B, Buguet S, Kester E, Baubet C, Tailhades P (1999) Cation valencies and distribution in the spinels CoxCuyMnzFeuO4+δ (δ ≥ 0) thin films studied by X-ray photoelectron spectroscopy. Thin Solid Films 357(2):223–231
Geng QF, Zhao X, Gao XH, Liu G (2011) Sol–gel combustion-derived CoCuMnOx spinels as pigment for spectrally selective paints. J Am Ceram Soc 94(3):827–832
Ma P, Geng Q, Gao X, Yang S, Gang L (2016) Aqueous solution-chemical derived spinel Cu1.5Mn1.5O4 thin film for solar absorber application. Mater Lett 179:170–174
Papavasiliou J, Avgouropoulos G, Ioannides T (2007) Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J Catal 251(1):7–20
Terayama K, Ikeda M (2007) Study on thermal decomposition of MnO2 and Mn2O3 by thermal analysis. Mater Trans 24(11):754–758
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254(8):2441–2449
Li F, Liu X, Yang Q, Liu J, Evans DG, Duan X (2005) Synthesis and characterization of Ni1−xZnxFe2O4 spinel ferrites from tailored layered double hydroxide precursors. Mater Res Bull 40(8):1244–1255
Kumar L, Kar M (2011) Influence of Al3+ ion concentration on the crystal structure and magnetic anisotropy of nanocrystalline spinel cobalt ferrite. J Magn Magn Mater 323(15):2042–2048
Thang PD, Rijnders G, Blank DHA (2005) Spinel cobalt ferrite by complexometric synthesis. J Magn Magn Mater 295(3):251–256
Acknowledgement
The authors gratefully acknowledge funding support from the Fundamental Research Funds for the Central Universities and support from Sichuan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, H., Gao, Z., Li, X. et al. Synthesis and characterization of CuFeMnO4 prepared by co-precipitation method. J Mater Sci 53, 3581–3589 (2018). https://doi.org/10.1007/s10853-017-1800-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1800-4