Abstract
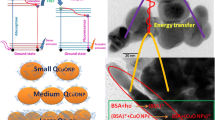
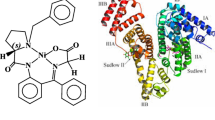
The interaction of covellite hexagonal phase of copper sulphide nanoparticles (CuS NPs) with bovine serum albumin (BSA) was examined systematically by using fluorescence, UV–visible, circular dichroism (CD), Fourier transform infrared (FTIR), dynamic light scattering (DLS) and molecular modelling techniques. Electrochemical method was studied to further confirm the interaction of BSA with CuS NPs. The results of fluorescence studies demonstrated that fluorescence of BSA was quenched by CuS NPs via a static quenching mechanism. The negative values of thermodynamic parameters (ΔG, ΔH and ΔS) indicated that the binding process is spontaneous, exothermic and van der Waals force or hydrogen bonding plays major roles in the interaction of CuS NPs with BSA. The interaction of CuS NPs with Trp residue was established by synchronous studies, and competitive binding studies revealed that Trp-212 of subdomain IIA was involved in the interaction with these nanoparticles. Further, the efficiency of energy transferred and the distance between fluorophore (BSA) and acceptor (CuS NPs) were calculated using Forster’s resonance energy transfer theory. The results of UV–visible, CD, FTIR and DLS revealed that the CuS NPs interact with BSA by inducing the conformational changes in secondary structure and reducing the α-helix content of BSA. Molecular modelling studies suggested that CuS NPs bind to site I of sub domain IIA of BSA. The results of spectroscopic and molecular docking studies were complimented by the electrochemical techniques.








Similar content being viewed by others
References
Chithaiah PG, Vijayakumar G, Nagabhushana GP, Nagaraju G, Chandrappa GT (2014) Physica E 59:218–222
Chithaiah P, Chandrappa GT, Livage JP (2012) Inorg Chem 51:2241–2246
Guan J, Peng J, Jin X (2015) Anal Methods 7:5454–5461
Yuan DP, Huang GP, Zhang FP, Yin D, Wang L (2016) Electrochim Acta 203:238–245
Maji SK, Dutta AK, Bhadu GR, Paul P, Mondal A, Adhikary B (2013) J Mater Chem B 1:4127–4134
Radhakrishnan S, Kim HY, Kim B (2016) s. Sens Actu B 233:93–99
Cai L, Sun Y, Li W, Zhang W, Liu X, Dinga D, Xua N (2015) RSC Adv 5:98136–98143
Lu Y, Liu X, Wang W, Cheng J, Yan H, Tang C, Kim JK, Luo Y (2015) Sci Rep 5:16584-1-10
Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, Yang S, Wang J, Hu J (2011) ACS Nano 5:9761–9771
Li Y, Lu W, Huang Q, Huang M, Li C, Chen W (2010) Nanomedicine 5:1161–1171
Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J (2011) Adv Mater 23:3542–3547
Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, Yan B, Lu W (2014) ACS Nano 8:5670–5681
Ghosh S, Dey J (2015) J Phys Chem B 119:7804–7815
Ju P, Fan H, Liu T, Cui L, Ai SWu, Wu X (2011) Biol Trans Elem Res 144:1405–1418
Ju P, Fan H, Liu T, Cui L, Ai S (2011) J Luminesc 131:1724–1730
Weser JK, Seller ED (1976) New Eng J Med 294:311–316
Lynch I, Dawson KA (2008) Nanotoday 3:41–47
Esfandfar P, Falahati M, Saboury AA (2016) J Biomol Struct Dyn 34:1962–1968
Zhang C, Fu YY, Zhang X, Yu C, Zhaoc Y, Sun SK (2015) Dalton Trans 44:13112–13118
Han L, Zhang Y, Chen XW, Shu Y, Wang JH (2016) J Mater Chem B 4:105–112
Mallappa M, Shivaraj Y, Nagaraju K, Vusa CSR (2016) Sens Actuators, A 248:104–113
Ishtikhara M, Alib MS, Attab AM, Al-Lohedanb H, Badrc G, Khana RH (2016) Int J Biol Macromol 82:844–855
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) J Comput Chem 25:1605–1612
Trott Oleg, Olson Arthur J, Vina AutoDock (2010) Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Krishna RH, Nagabhushana BM, Nagabhushana H, Murthy NS, Sharma SC, Shivakumara C, Chakradhar RPS (2013) J Phys Chem C 117:1915–1924
Jalali F, Dorraji PS, Mahdiuni H (2014) J Luminesc 148:347–352
Shahabadi N, Maghsudi M, Rouhani S (2012) Food Chem 135:1836–1841
Kathiravan A, Paramaguru G, Renganathan R (2009) J Mol Struct 934:129–137
Zaidi N, Ahmad E, Rehan M, Rabbani G, Ajmal MR, Zaidi Y, Subbarao N, Khan RH (2013) J Phys Chem B 117:2595–2604
Khan AB, Khan JM, Ali MS, Khan RH, Din K (2012) Spectrochim. Acta A Mol Biomol. Spectrosc 97:119–124
Mariam J, Dongre PM, Kothari DC (2011) J Fluoresc 21:2193–2199
Ross PD, Subramanian S (1981) Biochemistry 20:3096–3102
Lloyd JBF, Evett IW (1977) Anal Chem 49:1710–1715
Shen GF, Liu TT, Wang Q, Jiang M, Shi JH (2015) J Photochem Photobiol B Biol 153:380–390
Sudlow G, Birkett DJ, Wade DN (1976) Mol Pharmacol 12:1052–1061
Iranfar H, Rajabi O, Salari R, Chamani J (2012) J Phys Chem B 116:1951–1964
Forster T (1948) Ann Phys 2:55–75
Jattinagoudar L, Meti M, Nandibewoor S, Chimatadar S (2016) Spectrochim Acta A Mole Biomol Spectrosc 156:164–171
Chatterjee S, Mukherjee TK (2014) Phys Chem Chem Phys 16:8400–8408
Rashidipour S, Naeeminejad S, Chamani J (2016) J Biomol Struct Dyn 34:57–77
Huanga S, Qiua H, Liub Y, Huanga C, Shenga J, Sua W (2015) Col Sur B Biointer 136:955–962
Chaturvedi SK, Ahmad E, Khan JM, Alam P, Ishtikhar M, Khan RH (2015) Mol BioSyst 11:307–316
Ahmad F, Zhou Y, Ling Z, Xianga Q, Zhouc X (2016) RSC Adv 6:35719–35730
Lian GQ, Ran L, Lei JF, Cheng TJ, Wei LL, Yi L (2009) Acta Phys Chim Sin 25:2147–2154
Wang J, Xiang C, Tian FF, Xu ZQ, Jiang FL, Liu Y (2014) RSC Adv 4:18205–18216
Zhang J, Li R, Jiang FL, Zhou B, Luo QY, Yu QLY, Han XL, Lin Y, He H, Liu Y, Wang YL (2014) Col Sur B Biointer 117:68–74
Huang S, Zhu F, Xiao Q, Liang Y, Zhou Q, Su W (2015) RSC Adv 5:42889–42902
Li DL, Zou XQ, Shen Q, Dong S (2007) J. Electrochem Commun 9:191–196
Acknowledgements
Shivaraj Yellappa acknowledge the Raman Fellowship received from University Grants Commission [F. No. 5-120/2016(IC)], India, Prof. Raghavan Varadarajan, Department of Molecular Biophysics Unit, Indian Institute of Science, Bengaluru, to provide a circular dichroism spectrophotometer instrument facility and Prof. Francis D’souza, Department of Chemistry, University of North Texas, USA for technical discussion. Thanks to Mr. Sandeep, Application specialist, Malvern-Aimil application centre for assisting the DLS studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahanthappa, M., Savanur, M.A., Puthusseri, B. et al. Spectroscopic and electrochemical studies on the molecular interaction between copper sulphide nanoparticles and bovine serum albumin. J Mater Sci 53, 202–214 (2018). https://doi.org/10.1007/s10853-017-1521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1521-8