Abstract
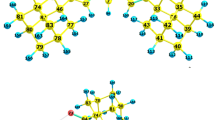
The hydrogen bonds and the conformations of calix[8]arene molecules with p-tert-butyl and p-1-adamantyl substituents were studied by infrared spectroscopy in different states. The conformations, the reactivity, the charge distribution and the IR spectra of the calixarenes were calculated by the DFT method with the PBE functional and a TZVP basis set. We compare the IR spectra of calix[8]arene molecules in the conformation of the pleated loop and the chair. The optimized geometry of the molecules reproduces the experimental X-ray data. The conformation chair is 20 kcal/mol less preferable than the conformation pleated loop. The conformation of a pleated loop is the most stable in the solid state and solution. Hydrogen bonds determine the stability of this structure. In the p-1-adamantylcalix[8]arene, stronger hydrogen bonds are realized compared to p-tert-butylcalix[8]arene. The observed IR spectra were interpreted using the calculated potential energy distribution with the quantum-chemical force constants. The theoretical absorption curves calculated for the pleated loop conformation correspond to the experimental IR spectra of the calix[8]arenes.







Similar content being viewed by others
References
Gutsche, C.D.: Calixarenes Revisited. The Royal Society of Chemistry, Cambridge (1998)
Vicens, J., Bohmer, V.: Calixarenes: A Versatile Class of Macrocyclic Compounds. Kluwer Academic Publishers, Boston (1991)
Konig, B., Fonseca, M.H.: Heteroatom-bridged calixarenes. Eur. J. Inorg. Chem. 2000, 2303–2310 (2000)
Opaprakasit, P., Scaroni, A., Painter, P.: Intramolecular hydrogen bonding and calixarene-like structures in p-cresol/formaldehyde resins. J. Mol. Struct. 570, 25–35 (2001)
Lutz, B.T.G., Astarloa, G., van der Maas, J.H., Janssen, R.G., Verboom, W., Reinhoudt, D.N.: Conformational isomerism and self-association of calixarene building blocs in non-polar solution solution studied by Fourier transform infrared spectrometry. Vib. Spectrosc. 10, 29–40 (1995)
Shokova, E.A., Khomich, E.V., Akhmetov, N.N., Vatsuro, I.M., Luzikov, YuN, Kovalev, V.V.: Synthesis and conformations of adamantylated calix[5]- and -[6]arenes. Russ. J. Org. Chem. 39, 368–383 (2003)
Czugler, M., Tisza, S., Speier, G.: Versatility in inclusion hosts. Unusual conformation in the crystal structure of the p-t-butylcalix[8]arene: pyridine (1:8) clathrate. J. Incl. Phenom. 11, 323–331 (1991)
Schatz, J., Schildbach, F., Lentz, A., Rastatter, S., Schilling, J., Dormann, J., Ruoff, A., Debaerdemaeker, T.Z.: The inclusion of carbon disulfide in p-tert-butylcalix[4]- and [6]arene-A combined crystallographic and vibrational spectroscopic study. Teil B 55, 213–221 (2000)
Billes, F., Mohammed-Ziegler, I.: Ab initio equilibrium geometry and vibrational spectroscopic study of 25,26,27,28-tetrahydroxycalix[4]arene. Supramol. Chem. 14, 451–459 (2002)
Katsyuba, S.A., Kovalenko, V.I., Chernova, A.V., Vandyukova, E.E., Zverev, V.V., Shagidullin, R.R., Antipin, I.S., Solovieva, S.E., Stoikov, I., Konovalov, A.I.: Vibrational spectra, co-operative intramolecular hydrogen bonding and conformations of calix[4]arene and thiacalix[4]arene molecules and their para-tert-butyl derivatives. Org. Biomol. Chem. 3, 2558–2565 (2005)
Furer, V.L., Borisoglebskaya, E.I., Kovalenko, V.I.: Band intensity in the IR spectra and conformations of calix[4]arene and thiacalix[4]arene. Spectrochim. Acta. 61, 355–359 (2005)
Furer, V.L., Borisoglebskaya, E.I., Zverev, V.V., Kovalenko, V.I.: The hydrogen bonding and conformations of p-tert-butylcalix[4]arene as studied by IR spectroscopy and by DFT calculations. Spectrochim. Acta 62, 483–493 (2005)
Furer, V.L., Borisoglebskaya, E.I., Zverev, V.V., Kovalenko, V.I.: DFT and IR spectroscopic analysis of p-tert-butylthiacalix[4]arene. Spectrochim. Acta 63, 207–212 (2006)
Furer, V.L., Potapova, L.I., Kovalenko, V.I.: DFT study of hydrogen bonding and IR spectra of calix[6]arene. J. Mol. Struct. 1128, 439–447 (2017)
Brzezinski, B., Urjasz, H., Zundel, G.: Cyclic hydrogen-bonded system with large proton polarizability in calixarenes. An FTIR study. J. Phys. Chem. 100, 9021–9023 (1996)
Konishi, H., Ohata, K., Morikawa, O., Kobayashi, K.: Calix[6]resorcinearenes: the first examples of [16]metacyclophanes derived from resorcinols. J. Chem. Soc. Chem. Commun. 56, 309–310 (1995)
Lang, J., Deckerova, V., Czernek, J., Lhotak, P.: Dynamics of circular hydrogen bond array in calix[4]arene in a nonpolar solvent: a nuclear magnetic resonance study. J. Chem. Phys. 122, 044506–044510 (2005)
Billes, F., Mohammed-Ziegler, I.: Transportation behavior of alkali ions through a cell membrane ion channel. A quantum chemical description of a simplified isolated model. J. Mol. Model 18, 3627–3637 (2012)
Cerioni, G., Biali, S., Rappoport, Z.: Hydrogen bonding in calix[n]arenes. A preliminary 17O NMR study. Tetrahedron Lett. 37, 5797–5800 (1996)
Kovalenko, V.I., Maklakov, L.I., Borisoglebskaya, E.I., Potapova, L.I., Shokova, E.А., Vatsuro, I.М., Kovalev, V.V.: Intramolecular co-operative hydrogen bond in calix[n]arenes (n = 4, 6, 8) bearing bulky substituents. Russ. Chem. Bull. Int. Ed. 56, 1103–1109 (2007)
Athar, M., Lone, M.Y., Jha, P.C.: Theoretical assessment of calix[n]arene as drug carries for second generation tyrosine kinase inhibitors. J. Mol. Liq. 247, 448–455 (2017)
Galindo-Murillo, R., Angilar-Suarez, L.E., Barroso-Flores, J.: A mixed DFT-MD methodology for the in silico development of drug releasing macrocycles. Calix and thia-calix[N]arenes as carriers for Bosutinib and Sorafenib. J. Comput. Chem. 37, 940–946 (2016)
Shahabi, M., Raissi, H.: Assessment of solvent effects on the inclusion behavior of pyrazinamide drug into cyclic peptide based nanotubes as novel drug delivery vehicles. J. Mol. Liq. 268, 326 (2018)
Oguz, M., Bhatti, A.A., Karakurt, S., Aktas, M., Yilmaz, M.: New water soluble Hg2+ selective fluorescent calix[4]arenes: synthesis and applications in living cells imaging. Spectrochim. Acta 171, 340–345 (2017)
Lubitov, I.E., Shokova, E.А., Kovalev, V.V.: New class of host molecules. p-1-Adamantylcalix[8]arenes. Synlett 1993, 647–648 (1993)
Perdew, J.P., Burke, K., Erznzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
Erznzerhof, M., Scuseria, G.E.: Assessment of the Perdew–Burke–Erzernhof exchange-correlation functional. J. Chem. Phys. 110, 5029–5036 (1999)
Laikov, D.N.: Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 281, 151–156 (1997)
Laikov, D.N., Ustynyuk, YuA: PRIRODA-04: a quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. Int. Ed. 54, 820–826 (2005)
Sipachev, V.A.: Calculation of shrinkage corrections in harmonic approximation. J. Mol. Struct. (Theochem) 121, 143–151 (1985)
Glendening, E.D., Landis, C.R., Weinhold, F.: Natural bond orbital methods. Comput. Mol. Sci. 2, 1–42 (2012)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09 Revision C.01. Gaussian Inc., Wallingford (2010)
Parr, R.G., Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983)
Steinbach, C., Farnik, M., Ettischer, I., Siebers, J., Buck, U.: Isomeric transitions in size-selected methanol hexamers probed by OH-stretch spectroscopy. Phys. Chem. Chem. Phys. 8, 2752–2758 (2006)
Atwood, J.L., Barbour, L.J., Jerga, A.: Organization of the interior of molecular capsules by hydrogen bonding. Proc. Nat. Acad. Sci. 99, 4837–4841 (2002)
Atwood, J.L., Barbour, L.J., Heaven, M.W., Raston, C.L.: C60 and C70 compounds in the pincerlike jaws of calix[6]arene. Angew. Chem. 37, 981–983 (1998)
Wiberg, K.A.: Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968)
Novikov, A.N., Bacherikov, V.A., Shapiro, YuE, Gren, A.I.: Ab initio and density functional studies of cooperative hydrogen bonding in calix [4]-and calix [6]arenes. J. Struct. Chem. 47, 1003–1015 (2006)
Ugozzoli, F., Andretti, G.D.: Symbolic representation of the molecular conformation of calixarenes. J. Incl. Phenom. 13, 337–348 (1992)
Acknowledgement
Thanks to Prof. Dr. I.S. Antipin and Prof. Dr. S.E. Solovieva for kindly prepared p-tert-butylcalix[8]arene.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Furer, V.L., Potapova, L.I., Vatsouro, I.M. et al. Study of conformation and hydrogen bonds in the p-1-adamantylcalix[8]arene by IR spectroscopy and DFT. J Incl Phenom Macrocycl Chem 95, 63–71 (2019). https://doi.org/10.1007/s10847-019-00916-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00916-8