Abstract
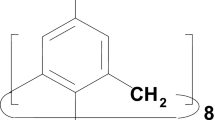
The IR spectra of p-(3-carboxy-1-adamantyl)-calix[4]arene (AdC4A) were studied. Using IR spectroscopy, it has been shown that in calixarene in dilute solution in CCl4, there was no free hydroxyl absorption band. The hydroxyl group band was characterized by a very low frequency, which indicates a strong intramolecular hydrogen bond on the lower rim of the calixarene molecules. On the upper rim of the calixarene, the carboxyl groups form cyclic dimer or tetramer complexes via intermolecular hydrogen bonds. The cone conformation persists, but there is a mutual influence of the hydrogen bonds along the top and bottom rims of the calixarene molecule. The structure with dimeric hydrogen bonds between the carboxyl groups is 16.5 KJ/mol more preferable than the structure with tetrameric cyclic hydrogen bonds for AdC4A. The reactivity depends on the type of association on the upper rim, whether these are hydrogen-bonded dimers or cyclic tetrameric association.

.






Similar content being viewed by others
References
Vicens J, Harrowfield J, Baklouti L (eds) (2007) Calixarenes in the nanoworld. Springer, Dordrecht
Neri P., Sessler J., Wang M.-X.(Ed.).: Calixarenes and beyond. Springer International Publishing, Switzerland, (2016)
Pellet-Rostaing S, Chitry F, Nicod L, Lemaire M (2001) Synthesis and complexation properties of 1,3-alternate calix[4]arene-bis(crown-6)derivatives. J Chem Soc Perkin Trans 2:1426–1432
Gutsche CD, Iftikhar A (1988) Calixarenes. 23. The complexation and catalytic properties of water soluble calixarene. Tetrahedron 44:4689–4694
Konig B, Fonseca MH (2000) Heteroatom-bridged calixarenes. Eur J Inorg Chem 2000:2303–2310
Opaprakasit P, Scaroni A, Painter P (2001) Intramolecular hydrogen bonding and calixarene-like structures in p-cresol/formaldehyde resins. J Mol Struct 570:25–35
Bellamy L (1975) The infra-red spectra of complex molecules. Springer
Shokova EA, Motornaya AE, Shestakova AK, Kovalev VV (2004) p-(3-Carboxy- and 3-carboxymethyl-1-1adamantyl)calix[4]arenes: synthesis and arming with amino acid units. Tetrahedron Lett 45:6465–6469
Katsyuba SA, Kovalenko VI, Chernova AV, Vandyukova EE, Zverev VV, Shagidullin RR, Antipin IS, Solovieva SE, Stoikov I, Konovalov AI (2005) Vibrational spectra, co-operative intramolecular hydrogen bonding and conformations of calix[4]arene and thiacalix[4]arene molecules and their para-tert-butyl derivatives. Org Biomol Chem 3:2558–2565
Furer VL, Borisoglebskaya EI, Kovalenko VI (2005) Band intensity in the IR spectra and conformations of calix[4]arene and thiacalix[4]arene. Spectrochim Acta 61:355–359
Furer VL, Borisoglebskaya EI, Zverev VV, Kovalenko VI (2005) The hydrogen bonding and conformations of p-tert-butylcalix[4]arene as studied by IR spectroscopy and by DFT calculations. Spectrochim Acta 62:483–493
Furer VL, Borisoglebskaya EI, Zverev VV, Kovalenko VI (2006) DFT and IR spectroscopic analysis of p-tert-butylthiacalix[4]arene. Spectrochim Acta 63:207–212
Furer VL, Potapova LI, Kovalenko VI (2017) DFT study of hydrogen bonding and IR spectra of calix[6]arene. J Mol Struct 1128:439–447
Furer VL, Potapova LI, Vatsouro IM, Kovalev VV, Shokova EA, Kovalenko VI (2019) Study of conformation and hydrogen bonds in the p-1-adamantylcalix[8]arene and IR spectroscopy and DFT. J Incl Phenom 95:63–71
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (eds) (2010) Gaussian 09 Revision C.01. Gaussian Inc., Wallingford
Sipachev VA (1985) Calculation of shrinkage corrections in harmonic approximation. J Mol Struct (THEOCHEM) 121:143–151
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. Comput Mol Sci 2:1–42
Wiberg KA (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron. 24:1083–1096
Ugozzoli F, Andretti GD (1992) Symbolic representation of the molecular conformation of calixarenes. J Incl Phenom 13:337–348
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Acknowledgments
The authors are grateful to the Assigned Spectral-Analytical Center of FRC Kazan Scientific Center of RAS for technical assistance in research.
Funding
Contributions to research were funded by the state assignment to: Federal Scientific Center “Scientific Research Institute for System Analysis of the RAS” (CDV) and A.E. Arbuzov Institute of Organic and Physical Chemistry RAS (V.I.K.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 337 kb)
Rights and permissions
About this article
Cite this article
Furer, V.L., Potapova, L.I., Chachkov, D.V. et al. Study of p-(3-carboxy-1-adamantyl)-calix[4]arene with hydrogen bonds along the upper and lower rim by IR spectroscopy and DFT. J Mol Model 26, 179 (2020). https://doi.org/10.1007/s00894-020-04441-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04441-1