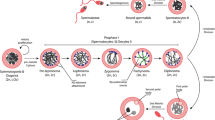
Abstract
Telomeres are located at the ends of linear chromosomes and play a critical role in maintaining genomic stability by preventing premature activation of DNA repair mechanisms. Because of exposure to various genotoxic agents, telomeres can undergo shortening and genetic changes. In mammalian cells, the basic DNA repair mechanisms, including base excision repair, nucleotide excision repair, double-strand break repair, and mismatch repair, function in repairing potential damages in telomeres. If these damages are not repaired correctly in time, the unfavorable results such as apoptosis, cell cycle arrest, and cancerous transition may occur. During lifespan, mammalian somatic cells, male and female germ cells, and preimplantation embryos experience a number of telomeric damages. Herein, we comprehensively reviewed the crosstalk between telomeres and the DNA repair mechanisms in the somatic cells, germ cells, and embryos. Infertility development resulting from possible defects in this crosstalk is also discussed in the light of existing studies.




Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20(1):15–30.
Doksani Y. The response to DNA damage at telomeric repeats and ıts consequences for telomere function. Genes (Basel). 2019;10(4):318.
Longhese MP. DNA damage response at functional and dysfunctional telomeres. Genes Dev. 2008;22(2):125–40.
Doksani Y, de Lange T. The role of double-strand break repair pathways at functional and dysfunctional telomeres. Cold Spring Harb Perspect Biol. 2014;6(12):a016576.
Tire B, Ozturk S. Potential effects of assisted reproductive technology on telomere length and telomerase activity in human oocytes and early embryos. J Ovarian Res. 2023;16(1):130.
O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11(3):171–81.
Tan J, Lan L. The DNA secondary structures at telomeres and genome instability. Cell Biosci. 2020;10:47.
Kent T, Clynes D. Alternative lengthening of telomeres: lessons to be learned from telomeric DNA double-strand break repair. Genes (Basel). 2021;12(11):1734.
Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019;177:37–45.
Lopes AC, Oliveira PF, Sousa M. Shedding light into the relevance of telomeres in human reproduction and male factor infertilitydagger. Biol Reprod. 2019;100(2):318–30.
Zhang Q, Kim NK, Feigon J. Architecture of human telomerase RNA. Proc Natl Acad Sci U S A. 2011;108(51):20325–32.
Wyatt HD, West SC, Beattie TL. InTERTpreting telomerase structure and function. Nucleic Acids Res. 2010;38(17):5609–22.
Armstrong L, et al. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech Dev. 2000;97(1-2):109–16.
Liu L, et al. Telomere lengthening early in development. Nat Cell Biol. 2007;9(12):1436–41.
Heaphy CM, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–15.
Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–30.
Mao P, et al. Homologous recombination-dependent repair of telomeric DSBs in proliferating human cells. Nat Commun. 2016;7:12154.
Fasching CL, et al. DNA damage induces alternative lengthening of telomeres (ALT) associated promyelocytic leukemia bodies that preferentially associate with linear telomeric DNA. Cancer Res. 2007;67(15):7072–7.
Azzalin CM, et al. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801.
Rocca MS, Foresta C, Ferlin A. Telomere length: lights and shadows on their role in human reproduction. Biol Reprod. 2019;100(2):305–17.
Kordowitzki P, et al. Dynamics of telomeric repeat-containing RNA expression in early embryonic cleavage stages with regards to maternal age. Aging (Albany NY). 2020;12(16):15906–17.
Ozturk S. Telomerase activity and telomere length in male germ cells. Biol Reprod. 2015;92(2):53.
Talibova G, Bilmez Y, Ozturk S. DNA double-strand break repair in male germ cells during spermatogenesis and its association with male infertility development. DNA Repair (Amst). 2022;118:103386.
Antunes DM, et al. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32(11):1685–90.
Stindl R. The paradox of longer sperm telomeres in older men’s testes: a birth-cohort effect caused by transgenerational telomere erosion in the female germline. Mol Cytogenet. 2016;9:12.
de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–10.
Imran SAM, et al. The ıntra- and extra-telomeric role of TRF2 in the DNA damage response. Int J Mol Sci. 2021;22(18):9900.
Doksani Y, et al. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013;155(2):345–56.
Feuerhahn S, et al. No DDRama at chromosome ends: TRF2 takes centre stage. Trends Biochem Sci. 2015;40(5):275–85.
Ruis P, Boulton SJ. The end protection problem-an unexpected twist in the tail. Genes Dev. 2021;35(1-2):1–21.
Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7(7):712–8.
van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92(3):401–13.
Karlseder J, et al. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283(5406):1321–5.
Lin J, Epel E. Stress and telomere shortening: ınsights from cellular mechanisms. Ageing Res Rev. 2022;73:101507.
Buscemi G, et al. The shelterin protein TRF2 inhibits Chk2 activity at telomeres in the absence of DNA damage. Curr Biol. 2009;19(10):874–9.
Sfeir A, et al. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327(5973):1657–61.
de Lange T. Shelterin-mediated telomere protection. Annu Rev Genet. 2018;52:223–47.
Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068–71.
Wu L, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126(1):49–62.
Yang Q, Zheng YL, Harris CC. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol. 2005;25(3):1070–80.
Uysal F, et al. Decreased expression of TERT and telomeric proteins as human ovaries age may cause telomere shortening. J Assist Reprod Genet. 2021;38(2):429–41.
Pirzada RH, et al. Role of TRF2 and TPP1 regulation in idiopathic recurrent pregnancy loss. Int J Biol Macromol. 2019;127:306–10.
Yokoyama H, et al. Preferential binding to branched DNA strands and strand-annealing activity of the human Rad51B, Rad51C, Rad51D and Xrcc2 protein complex. Nucleic Acids Res. 2004;32(8):2556–65.
Doksani Y, de Lange T. Telomere-ınternal double-strand breaks are repaired by homologous recombination and PARP1/Lig3-dependent end-joining. Cell Rep. 2016;17(6):1646–56.
Bhargava R, Onyango DO, Stark JM. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet. 2016;32(9):566–75.
Kazda A, et al. Chromosome end protection by blunt-ended telomeres. Genes Dev. 2012;26(15):1703–13.
Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34.
Mehta A, Haber JE. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb Perspect Biol. 2014;6(9):a016428.
Scully R, et al. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019;20(11):698–714.
West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003;4(6):435–45.
Henson JD, et al. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21(4):598–610.
Britton S, Coates J, Jackson SP. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol. 2013;202(3):579–95.
Yue X, et al. DNA-PKcs: a multi-faceted player in DNA damage response. Front Genet. 2020;11:607428.
Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72(1):131–42.
Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66(6):801–17.
Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211.
Sfeir A, Symington LS. Microhomology-mediated end joining: a back-up survival mechanism or dedicated pathway? Trends Biochem Sci. 2015;40(11):701–14.
Chang HHY, et al. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18(8):495–506.
Zhu Z, et al. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134(6):981–94.
Garcia-Rodriguez A, et al. DNA damage and repair in human reproductive cells. Int J Mol Sci. 2018;20(1):31.
Musson R, et al. DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies. Hum Reprod Update. 2022;28(3):376–99.
Martin JH, et al. DNA damage and repair in the female germline: contributions to ART. Hum Reprod Update. 2019;25(2):180–201.
Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117(1-2):15–23.
Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 2012;22(11):989–94.
Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272(2):483–96.
Huang Y, Roig I. Genetic control of meiosis surveillance mechanisms in mammals. Front Cell Dev Biol. 2023;11:1127440.
Jaroudi S, et al. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24(10):2649–55.
Veitia RA. Primary ovarian insufficiency, meiosis and DNA repair. Biomed J. 2020;43(2):115–23.
Huang C, Guo T, Qin Y. Meiotic recombination defects and premature ovarian ınsufficiency. Front Cell Dev Biol. 2021;9:652407.
Butts S, et al. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94(12):4835–43.
Toupance S, et al. Ovarian telomerase and female fertility. Biomedicines. 2021;9:7.
Fattet AJ, et al. Telomere length in granulosa cells and leukocytes: a potential marker of female fertility? A systematic review of the literature. J Ovarian Res. 2020;13(1):96.
Dinger Y, et al. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scand J Clin Lab Invest. 2005;65(8):721–8.
Pedroso DCC, et al. Telomere length and telomerase activity in ımmature oocytes and cumulus cells of women with polycystic ovary syndrome. Reprod Sci. 2020;27(6):1293–303.
Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2003;6(1):84–96.
Derijck A, et al. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17(13):1922–37.
Treff NR, et al. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161.
Goedecke W, et al. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23(2):194–8.
Gouraud A, et al. “Breaking news” from spermatids. Basic Clin Androl. 2013;23:11.
Ahmed EA, Scherthan H, de Rooij DG. DNA double strand break response and limited repair capacity in mouse elongated spermatids. Int J Mol Sci. 2015;16(12):29923–35.
Ahmed EA, et al. Ku70 and non-homologous end joining protect testicular cells from DNA damage. J Cell Sci. 2013;126(Pt 14):3095–104.
Ahmed EA, Rosemann M, Scherthan H. NHEJ contributes to the fast repair of radiation-induced DNA double-strand breaks at late prophase I telomeres. Health Phys. 2018;115(1):102–7.
Siderakis M, Tarsounas M. Telomere regulation and function during meiosis. Chromosome Res. 2007;15(5):667–79.
Roig I, et al. Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma. 2004;113(1):22–33.
Badie S, et al. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol. 2010;17(12):1461–9.
Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet. 2013;4:85.
Titus S, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21.
Jaco I, et al. Role of mammalian Rad54 in telomere length maintenance. Mol Cell Biol. 2003;23(16):5572–80.
Tarsounas M, et al. Telomere maintenance requires the RAD51D recombination/repair protein. Cell. 2004;117(3):337–47.
Espejel S, et al. Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86, or DNA-PKcs. J Cell Biol. 2004;167(4):627–38.
Li H, et al. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol. 2007;27(23):8205–14.
Iyama T, Wilson DM 3rd. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst). 2013;12(8):620–36.
Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev. 2006;106(2):253–76.
Petruseva IO, Evdokimov AN, Lavrik OI. Molecular mechanism of global genome nucleotide excision repair. Acta Naturae. 2014;6(1):23–34.
Wienholz F, Vermeulen W, Marteijn JA. Amplification of unscheduled DNA synthesis signal enables fluorescence-based single cell quantification of transcription-coupled nucleotide excision repair. Nucleic Acids Res. 2017;45(9):e68.
Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–65.
Wang S, et al. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci U S A. 2010;107(41):17639–44.
Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31(3):309–19.
Zhu XD, et al. ERCC1/XPF removes the 3’ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12(6):1489–98.
de Lange T. Telomere biology and DNA repair: enemies with benefits. FEBS Lett. 2010;584(17):3673–4.
Rochette PJ, Brash DE. Human telomeres are hypersensitive to UV-induced DNA damage and refractory to repair. PLoS Genet. 2010;6(4):e1000926.
Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci U S A. 1995;92(1):258–62.
Fouquerel E, Opresko PL. Convergence of The nobel fields of telomere biology and DNA repair. Photochem Photobiol. 2017;93(1):229–37.
Wu Y, Mitchell TR, Zhu XD. Human XPF controls TRF2 and telomere length maintenance through distinctive mechanisms. Mech Ageing Dev. 2008;129(10):602–10.
Ting AP, et al. Telomere attrition and genomic instability in xeroderma pigmentosum type-b deficient fibroblasts under oxidative stress. J Cell Mol Med. 2010;14(1-2):403–16.
Gopalakrishnan K, et al. Hydrogen peroxide induced genomic instability in nucleotide excision repair-deficient lymphoblastoid cells. Genome Integr. 2010;1(1):16.
Stout GJ, Blasco MA. Telomere length and telomerase activity impact the UV sensitivity syndrome xeroderma pigmentosum C. Cancer Res. 2013;73(6):1844–54.
Batenburg NL, et al. Cockayne syndrome group B protein interacts with TRF2 and regulates telomere length and stability. Nucleic Acids Res. 2012;40(19):9661–74.
Tan J, et al. An R-loop-initiated CSB-RAD52-POLD3 pathway suppresses ROS-induced telomeric DNA breaks. Nucleic Acids Res. 2020;48(3):1285–300.
Hagelstrom RT, et al. Hyper telomere recombination accelerates replicative senescence and may promote premature aging. Proc Natl Acad Sci U S A. 2010;107(36):15768–73.
Wallace SS. Base excision repair: a critical player in many games. DNA Repair (Amst). 2014;19:14–26.
Kim YJ, Wilson DM 3rd. Overview of base excision repair biochemistry. Curr Mol Pharmacol. 2012;5(1):3–13.
Schormann N, Ricciardi R, Chattopadhyay DJPs. Uracil-DNA glycosylases—structural and functional perspectives on an essential family of DNA repair enzymes. Protein Sci. 2014;23(12):1667–85.
Otterlei M, et al. Post-replicative base excision repair in replication foci. EMBO J. 1999;18(13):3834–44.
Hegde ML, et al. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J Biol Chem. 2008;283(40):27028–37.
Krokan HE, Bjoras M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012583.
Svilar D, et al. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. 2011;14(12):2491–507.
Jia P, Her C, Chai W. DNA excision repair at telomeres. DNA Repair (Amst). 2015;36:137–45.
von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–44.
Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–9.
Kordowitzki P. Oxidative stress induces telomere dysfunction and shortening in human oocytes of advanced age donors. Cells. 2021;10(8):1866.
Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40(15):4763–8.
Opresko PL, et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell. 2004;14(6):763–74.
Fouquerel E, et al. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol Cell. 2019;75(1):117–130 e6.
Wang Z, et al. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6(5):e1000951.
Rhee DB, et al. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst). 2011;10(1):34–44.
Vallabhaneni H, et al. Defective repair of uracil causes telomere defects in mouse hematopoietic cells. J Biol Chem. 2015;290(9):5502–11.
Benoff S, et al. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol Med. 2009;15(7-8):248–62.
Vallabhaneni H, et al. Defective repair of oxidative base lesions by the DNA glycosylase Nth1 associates with multiple telomere defects. PLoS Genet. 2013;9(7):e1003639.
Dejardin J, Kingston RE. Purification of proteins associated with specific genomic loci. Cell. 2009;136(1):175–86.
Lee OH, et al. Genome-wide YFP fluorescence complementation screen identifies new regulators for telomere signaling in human cells. Mol Cell Proteomics. 2011;10(2):M110 001628.
Madlener S, et al. Essential role for mammalian apurinic/apyrimidinic (AP) endonuclease Ape1/Ref-1 in telomere maintenance. Proc Natl Acad Sci U S A. 2013;110(44):17844–9.
Opresko PL, et al. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33(4):1230–9.
Li M, et al. APE1 deficiency promotes cellular senescence and premature aging features. Nucleic Acids Res. 2018;46(11):5664–77.
Jiricny J. Postreplicative mismatch repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012633.
Ioannou D, et al. Impact of sperm DNA chromatin in the clinic. J Assist Reprod Genet. 2016;33(2):157–66.
Amaral-Silva GK, et al. Mismatch repair system proteins in oral benign and malignant lesions. J Oral Pathol Med. 2017;46(4):241–5.
Martin-Lopez JV, Fishel R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam Cancer. 2013;12(2):159–68.
Brown MW, et al. Dynamic DNA binding licenses a repair factor to bypass roadblocks in search of DNA lesions. Nat Commun. 2016;7:10607.
Pecina-Slaus N, et al. Mismatch repair pathway, genome stability and cancer. Front Mol Biosci. 2020;7:122.
Siegl-Cachedenier I, et al. Deficient mismatch repair improves organismal fitness and survival of mice with dysfunctional telomeres. Genes Dev. 2007;21(17):2234–47.
Basheva EA, Bidau CJ, Borodin PM. General pattern of meiotic recombination in male dogs estimated by MLH1 and RAD51 immunolocalization. Chromosome Res. 2008;16(5):709–19.
Segui N, et al. Telomere length and genetic anticipation in Lynch syndrome. PLoS One. 2013;8(4):e61286.
Mendez-Bermudez A, Royle NJ. Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Hum Mutat. 2011;32(8):939–46.
Rampazzo E, et al. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer. 2010;102(8):1300–5.
Campbell MR, et al. Msh2 deficiency leads to chromosomal abnormalities, centrosome amplification, and telomere capping defect. Oncogene. 2006;25(17):2531–6.
Author information
Authors and Affiliations
Contributions
SO, BT, and GT designed the study. BT and GT wrote the manuscript and created all the figures. SO critically read and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Betul Tire and Gunel Talibova contributed to the manuscript equally.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tire, B., Talibova, G. & Ozturk, S. The crosstalk between telomeres and DNA repair mechanisms: an overview to mammalian somatic cells, germ cells, and preimplantation embryos. J Assist Reprod Genet 41, 277–291 (2024). https://doi.org/10.1007/s10815-023-03008-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-03008-2