Abstract
Purpose
Baboons are commonly utilized as an animal model for studies of human reproduction. However, folliculogenesis in this species has not been fully documented. The aim of this study was to assess follicle morphometry and expression of essential proteins involved in folliculogenesis in baboons.
Methods
Ovaries were recovered from four adult baboons and processed for histological evaluation and immunohistochemical analyses. Follicle proportion, follicle and oocyte diameter, theca layer thickness, number of granulosa cells, and follicle density were calculated. Immunohistochemical staining was also carried out for connexin 43 (Cx43), aromatase, and zona pellucida 3 (ZP3).
Results
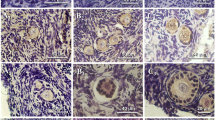
A total of 2221 follicles were counted and measured. Proportions of primordial, primary, secondary, small antral, and large antral follicles were 49, 26, 23, 1, and 1 %, respectively. The increase in follicle diameter was due not only to the increase in oocyte diameter but also to granulosa cell proliferation. Almost all antral follicles were positive for Cx43 (89.8 %), aromatase (84.8 %), and ZP3 (100 %). Most secondary follicles were positive for Cx43 (65 %) and ZP3 (64.5 %), and some primary follicles were positive only for Cx43. No primordial follicles stained positive in any of these immunohistochemical analyses. Only antral follicles showed aromatase activity.
Conclusions
On the basis of these results, we can conclude that folliculogenesis in baboons appears to be similar to that in humans, and this animal therefore constitutes a valuable model.






Similar content being viewed by others
References
Stevens VC. Some reproductive studies in the baboon. Hum Reprod Update. 1997;3:533–40.
Weatheall D. The use of non-human primates in research: a working group report chaired by Sir David Weatherall. 2006. http://www.acmedsci.ac.uk. Accessed 16 Oct 2015.
Donnez O, Van Langendonckt A, Defrère S, Colette S, Van Kerk O, Dehoux JP, et al. Induction of endometriosis nodules in an experimental baboon model mimicking human deep nodular lesions. Fertil Steril. 2013;99:783–9. e3.
Donnez O, Orellana R, Van Kerk O, Dehoux JP, Donnez J, Dolmans MM. Invasion process of induced deep nodular endometriosis in an experimental baboon model: similarities with collective cell migration? Fertil Steril. 2015;104:491–7. e2.
Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001.
Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirsfeld-Cytronn J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–97.
Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, et al. Successful vitrification and autografting of baboon (Papio anubis) ovarian tissue. Hum Reprod. 2013;28:2146–56.
Nyachieo A, Spiessens C, Chai DC, Kiulia NM, Willemen D, Mwenda JM, et al. Ovarian tissue cryopreservation by vitrification in olive baboons (Papio anubis): a pilot study. Gynecol Obstet Investig. 2013;75:157–62.
Díaz-Garcia C, Milenkovic M, Groth K, Dahm-Kähler P, Olausson M, Brännström M. Ovarian cortex transplantation in the baboon: comparison of four different intra-abdominal transplantation sites. Biol Reprod. 2011;84:689–97.
Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Regulation of oocyte microvilli development in the baboon fetal ovary by estrogen. Endocrinology. 2004;145:959–66.
Pepe GJ, Billiar RB, Albrecht ED. Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol Cell Endocrinol. 2006;247:41–6.
Burch MG, Li C, Albrecht ED, Pepe GJ. Developmental regulation of the expression of the transferrin receptor and Ki67 in oocytes of the baboon fetal ovary by estrogen. Endocrine. 2009;35:177–83.
Williams RF. Ovarian stimulation in nonhuman primate. In: Committee on the Basic Science Foundations of Medically Assisted Conception, Institute of Medicine and National Research Council, editor. Medically assisted conception—an agenda for research. Washington: National Academic Press; 1989. p. 117–29.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–55.
Griffin J, Benjamin R, Emery BR, Huang I, Peterson CM, Carrell DT. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J Exp Clin Assist Reprod. 2006;3:2.
Amorim CA, David A, Dolmans MM, Camboni A, Donnez J, Van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28:1157–65.
Gougeon A, Busso D. Morphologic and functional determinants of primordial and primary follicles in the monkey ovary. Mol Cell Endocrinol. 2000;163:33–42.
Almeida DV, Santos RR, Scalercion SR, Leão DL, Haritova A, Oskam IC, et al. Morphological and morphometrical characterization, and estimation of population of preantral ovarian follicles from senile common squirrel monkey (Saimiri sciureus). Anim Reprod Sci. 2012;134:210–5.
Duffy DM. Growth differentiation factor-9 is expressed by the primate follicle throughout the periovulatory interval. Biol Reprod. 2003;69:725–32.
Scalercio SR, Brito AB, Domingues SF, Santos RR, Amorim CA. Immunolocalization of growth, inhibitory, and proliferative factors involved in initial ovarian folliculogenesis from adult common squirrel monkey (Saimiri collinsi). Reprod Sci. 2015;22:68–74.
Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Müllerian inhibiting substance in the primate ovary. Reproduction. 2006;132:443–53.
Thomas FH, Telfer EE, Fraser HM. Expression of anti-Müllerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273281.
Sharara FI, Scott RT. Assessment of ovarian reserve. Is there still a role for ovarian biopsy? Hum Reprod. 2004;19:470–1.
Donnez J, Dolmans MM, Pellicer A, Díaz-Garcia C, Serrano MS, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13.
Westergaard CG, Byskov AG, Andersen CY. Morphometric characteristics of the primordial to primary follicle transition in the human ovary in relation to age. Hum Reprod. 2007;22:2225–31.
Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81:433–42.
Lundy T, Smith P, O’Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999;115:251–62.
Fair T. Follicular oocyte growth an acquisition of developmental competence. Anim Reprod Sci. 2003;78:203–16.
Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504.
David A, Dolmans MM, Van Langendonckt A, Donnez J, Amorim CA. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil Steril. 2011;95:1241–6.
Abir R, Fisch B, Jin S, Barnnet M, Kessler-Icekson G, Ao A. Expression of stem cell factor and its receptor in human fetal and adult ovaries. Fertil Steril. 2004;82:1235–43.
Tuck AR, Robker RL, Norman RJ, Tilley WD, Hickey TE. Expression and localization of c-kit and KITL in the adult human ovary. J Ovarian Res. 2015;8:31.
Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod. 2000;5:143–52.
Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–20.
Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol Cell Physiol. 2003;284:C880–7.
Wang HX, Tong D, El-gehani F, Tekpetey FR, Kidder GM. Connexin expression and gp junctional coupling in human cumulus cells: contribution to embryo quality. J Cell Mol Med. 2009;13:972–84.
Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88:399–413.
Johnson ML, Redmer DA, Reynolds LP, Grazul-Bilska AT. Expression of gap junctional proteins connexin 43, 32, and 26 throughout follicular development and atresia in cows. Endocrine. 1999;10:43–51.
Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40.
Ghazal S, Makaroc JLK, De Jonge CJ, Patriwio P. Egg transport and fertilization. 2014. http://www.glowm.com/section_view/heading/Egg%20Transport%20and%20Fertilization/item/316. Accessed 16 Oct 2015.
Hinsch E, Orhninger S, Schill WB, Hinsch KD. Species specificity of human and murine anti-ZP3 synthetic peptide antisera and use of the antibodies for localization and identification of ZP3 or ZPC domains of functional significance. Hum Reprod. 1999;14:419–28.
Gook DA, Edgar DH, Borg J, Martic M. Detection of zona pellucida proteins during human folliculogenesis. Hum Reprod. 2008;23:394–402.
Grootenhuis AJ, Philipsen HL, de Breet-Grijsbach JT, van Duin M. Immunocytochemical localization of ZP3 in primordial follicles of rabbit, marmoset, rhesus monkey and human ovaries using antibodies against human ZP3. J Reprod Fertil Suppl. 1996;50:43–54.
Bogner K, Hinsch KD, Nayudu P, Konrad L, Cassara C, Hinsch E. Localization and synthesis of zona pellucida proteins in the marmoset monkey (Callithrix jacchus) ovary. Mol Hum Reprod. 2004;10:481–8.
Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31.
Vegetti W, Alagna F. FSH and folliculogenesis: from physiology to ovarian stimulation. Reprod Biomed Online. 2006;12:684–94.
Bøtkjær JA, Jeppesen JV, Wissing ML, Kløverpris S, Oxvig C, Mason JI, et al. Pregnancy-associated plasma protein A in human ovarian follicles and its association with intrafollicular hormone levels. Fertil Steril. 2015;1294-301.e1.
McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–14.
Zhou J, Wang J, Penny D, Monget P, Arraztoa JA, Fogelson LJ, et al. Insulin-like growth factor binding protein 4 expression parallels luteinizing hormone receptor expression and follicular luteinization in the primate ovary. Biol Reprod. 2003;69:22–9.
Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73:473–87.
Acknowledgments
The authors thank Mira Hryniuk for reviewing the English language of the manuscript and Dolores Gonzalez and Olivier Van Kerk for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The present study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (grant 5/4/150/5 awarded to Marie-Madeleine Dolmans), the Fondation St Luc, and the Foundation Against Cancer, and donations from Mr Pietro Ferrero, Baron Albert Frère, and Viscount Philippe de Spoelberch.
Additional information
CA Amorim and CF Moya are joint first authors.
Capsule
These data suggest that folliculogenesis in baboons appears to be similar to that in humans, and this animal therefore constitutes a valuable model.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Immunohistochemical staining for KL. Stained granulosa cells from primordial and primary follicles and stromal cells (a). Stained granulosa cells from secondary (b), small antral (c), and large antral (d) follicles. Negative (e) and positive (f) controls. (JPG 340 kb)
Online Resource 2
Immunohistochemical staining for c-kit. Stained oolema, ooplasm and granulosa cell cytoplasm of primordial (a) and primary (b) follicles. Stained oolema and ooplasm of secondary (c) and small antral (d) follicles. Negative (e) and positive (f) controls. (JPG 468 kb)
Online Resource 3
Immunohistochemical staining for Cx43. Unstained primordial (a) and primary (b) follicles. Cx43 is localized between the granulosa cells and also between the granulosa cells and oocyte from secondary (c), small antral (d), and large antral (e) follicles. Negative (f) and positive (g) controls. (JPG 373 kb)
Online Resource 4
Immunohistochemical localization of ZP3. No immunoreactivity was observed in primordial (a) or primary (b) follicles, only surrounding the oocyte of secondary (red arrow), small antral (c) and large antral (d) follicles. Negative (e) and positive (f) controls. (JPG 536 kb)
Online Resource 5
Immunohistochemical staining for aromatase. Unstained primordial (a), primary (b) and secondary (c) follicles. Only granulosa cells (cytoplasm) from small antral (c) and large antral (d) follicles showed immunoreactivity for aromatase. Negative (e) and positive (f) controls. (JPG 276 kb)
Rights and permissions
About this article
Cite this article
Amorim, C.A., Moya, C.F., Donnez, J. et al. Morphometric characteristics of preantral and antral follicles and expression of factors involved in folliculogenesis in ovaries of adult baboons (Papio anubis). J Assist Reprod Genet 33, 617–626 (2016). https://doi.org/10.1007/s10815-016-0681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0681-9