Abstract
The oleaginous genera Nannochloropsis and Microchloropsis are recognized for their lipid accumulation capacity. Microalgal lipid accumulation is triggered by nitrogen starvation, negatively affecting photosynthesis and growth. Moreover, light and temperature play pivotal roles in microalgal physiology, lipid accumulation and composition. This study focuses on comparing the responses of eight microalgal strains from Nannochloropsis (N. oceanica Necton, N. oceanica IMET1, Nannochloropsis. sp. CCAP211/78, N. oculata, and N. limnetica) and Microchloropsis (M. gaditana CCFM01, M. gaditana CCMP526, and M. salina) to light, temperature, and nitrogen availability. Biomass, lipid content and productivities were monitored under different light intensities (150 (LL) and 600 μmol photons m−2 s−1 (HL)) and temperatures (15, 25, 30℃) under nitrogen (N-) starvation and replete conditions. Under N-starvation and HL, N. sp. exhibited the highest lipid content (59%) and productivity (0.069 g L-1 day-1), while N. oculata had the lowest lipid content (37.5%) and productivity (0.037 g L-1 day-1) among the eight strains. Notably, M. gaditana CCFM01 achieved the highest EPA content (4.7%), contrasting with N. oceanica IMET1 lowest EPA content (2.9%) under 150 μmol photons m−2 s−1 and N-repletion. The response to temperature fluctuations under LL was strain-dependent. Microchloropsis salina and M. gaditana CCFM01 demonstrated the highest and lowest lipid productivities (0.069 g L-1 day-1 and 0.022 g L-1 day-1, respectively) at 15℃ under N-starvation. Moreover, significant EPA accumulation across various strains was observed in N. oculata (5.7%) under N-repletion at 15°C, surpassing M. gaditana CCFM01 by 40%. Ultimately, the physiological responses to cultivation conditions vary markedly among microalgal strains, even within the same genus or species. This knowledge is essential for selecting suitable strains for the efficient microalgal lipid production industry.
Graphical Abstract
Optimi zing cultivation conditions for the maximal lipid production in Nannochloropsis andMicrochloropsis

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Microalgae are of interest because their potential to produce oils and a diverse array of high-value compounds, including unsaturated fatty acids, vitamins, pigments, carotenoids, polysaccharides, minerals, and other fine chemicals (Schulze et al. 2019; Abo-Shady et al. 2023) for an extensive range of applications, from biofuels to nutraceuticals (Abdel-Karim et al. 2020; Gheda et al. 2021). Their ability to thrive under adverse conditions without needing fertile land makes them suitable for sustainable cultivation without competing with food production (Sivagurulingam et al. 2022). Moreover, many microalgae can achieve high cell densities in marine or brackish water, significantly reducing water consumption and enhancing sustainability (Park and Lee 2016). They also require specific yet cost-effective growth conditions, allowing for rapid growth rates under high-light conditions.
However, there are challenges in microalgae lipid production and cultivation that hinder market introduction and expansion. These challenges encompass strain selection, strain improvement, the high cost of extracting essential components from algal biomass, and the adverse impacts of climate variations on microalgae production. Overcoming these hurdles will command technological advancements and scientific innovation in producing and processing microalgae-based lipids (Aratboni et al. 2019; Hanifzadeh and Nabati 2019).
The lipid content in microalgae typically ranges from 20 to 50% of the cell's dry weight under normal conditions (Gui et al. 2021) and under specific conditions it can reach up to 80% (Chisti 2007; Gui et al. 2021), comprising diverse molecules that vary in function from cellular structures to energy storage. Triacylglycerols (TAGs) are ideal for biofuel production and polyunsaturated fatty acids (PUFAs) like (eicosapentaenoic acid, EPA), which are commercially used in nutraceuticals production and aquaculture. Numerous strategies have been exploited to produce these lipids (El Shafay et al. 2021; Elshobary et al. 2022). These strategies encompass controlling nutrient availability (carbon, nitrogen and phosphorus), adjusting environmental conditions (pH, salinity, light intensity, and temperature) (Sibi et al. 2016; Gaignard et al. 2021; Morales et al. 2021), and implementing genetic and metabolic engineering approaches (Li-Beisson et al. 2015; Hulatt et al. 2020).
One of the widely adopted strategies for stimulating lipid accumulation, especially TAGs (energy storage) in microalgae, is nitrogen limitation. Nitrogen deficiency hampers microalgal growth and causes an energy imbalance by accumulating photosynthetic reductant power, leading to photo-oxidative damage of photosystems I and II (Juergens et al. 2015). To counteract this, TAG biosynthesis is stimulated to act as an alternative sink for the excess reductant power to protect the photosynthetic apparatus and enhance lipid accumulation functioning as energy reserves for cell survival (Klok et al. 2013; Karima et al. 2018; Maltsev et al. 2023). This approach has been shown to yield the highest lipid production in various species, including the genera Nannochloropsis and Microchloropsis (Converti et al. 2009; Jia et al. 2015; Huete-Ortega et al. 2018).
The choice of the most productive and resilient species for a given application is contingent upon their response to essential environmental factors like light and temperature (Sibi et al. 2016; Gaignard et al. 2021; Morales et al. 2021). Light is critical, impacting microalgae's photosynthetic and metabolic pathways. High-light intensities have enhanced lipid accumulation, influencing the equilibrium between the storage lipids and structural membrane (Nadzir et al. 2018; Metsoviti et al. 2020; Nzayisenga et al. 2020). It imposes a variation in the allocation of lipid classes, changes in fatty acid profile, and the increased percentage of shorter fatty acids associated with the formation of TAGs (Maltsev et al. 2021; Chin et al. 2023). The stress caused by higher light intensity under nitrogen deprivation further exacerbates the energy imbalance, leading to even greater TAG production (Solovchenko et al. 2014; El Shafay et al. 2021; Chin et al. 2023).
After light, temperature is another critical cultivation parameter that significantly influences all microalgal metabolic processes, growth, and biological composition, with the optimal temperature for a particular strain profoundly impacting culture productivity (Borowitzka 2016). Variability in culture environmental conditions, such as daily and seasonal temperature fluctuations, can lead to growth rate fluctuations and microalgae lipid accumulation (Sheng et al. 2011; Wei et al. 2015; Ippoliti et al. 2016). Deviations from the optimal temperatures can disrupt the metabolic balance by reducing the enzymatic processes associated with carbon and nitrogen assimilation, impact microalgal growth, affect membrane integrity, and alter the fatty acid profiles, particularly reducing unsaturated fatty acids which are vital for maintaining cellular membrane fluidity (Wei et al. 2015; Barati et al. 2019; Ferrer-Ledo et al. 2023).
The genera Nannochloropsis and Microchloropsis (formerly Nannochloropsis, Fawley et al. 2014) are some of the most attractive oleaginous microalgae for lipid production. Under stress conditions, they can produce storage carbohydrates (a β-1,3-glucan) and TAG as intracellular energy storage compounds (Morales et al. 2021). These genera comprise many different marine species (N. oceanica, N. oculata, N. granulata, M. gaditana and M. salina) and the freshwater N. limnetica, which notably accumulates over 60-70% TAG of its dry biomass under nitrogen starvation, which is drastically higher than the content of higher plants and other microalgae genera (Ma et al. 2014). In addition, they are natural sources of high-value PUFAs such as omega-3 fatty acids, including (EPA), which are commercially used in nutraceutical production and in aquaculture (Ryu et al. 2020). Moreover, these species have proven suitable for outdoor cultivation, offering versatile genetic platforms for the desired application (Naduthodi et al. 2021).
Nevertheless, significant variations have been reported between the different species and strains of Nannochloropsis and Microchloropsis in growth, lipid content, and lipid productivity, indicating the need for tailored cultivation strategies. For instance, Ma et al. (2014) observed that among 9 different strains of Nannochloropsis and Microchloropsis, N. oceanica IMET1 exhibited the fastest specific growth rate, lipid and TAG production. Moreover, N. sp. showed the highest fatty acid content and biomass concentration among the tested species (Benvenuti et al. 2014). Yet, it is worth noting that none of these studies were conducted under the same cultivation conditions, making direct species-to-species comparisons challenging. Additionally, they focused on how light and temperature conditions impact growth under nitrogen-replete conditions, leaving limited information on their influence on lipid accumulation during nitrogen starvation.
In this study, we explore the effects of light intensity, temperature, and their interaction with nitrogen availability on biomass and lipid production for eight strains from the genera Nannochloropsis (N. oceanica Necton, N. oceanica IMET1, N. sp. CCAP211/78, N. oculata, and N. limnetica) and Microchloropsis (M. gaditana CCFM01, M. gaditana CCMP526, M. salina).
Materials and methods
Algal strains, growth medium, and pre-cultivation conditions
Nannochloropsis oceanica Necton (was provided by NECTON, SA (Olhão, Portugal)), Nannochloropsis oceanica IMET1 was a kind gift from Prof. Jian Xu (Qingdao Institute for Bioenergy and Bioprocess Technology, Chinese Academy of Sciences). The other strains were from various culture collections: Microchloropsis gaditana CCFM-01 (Microalgae Collection of Fitoplancton Marino S.L., CCFM, Spain), Microchloropsis gaditana CCMP526 and Nannochloropsis limnetica (Culture Collection of National Center for Marine Algae and Microbiota, USA), Nannochloropsis sp. CCAP211/78 (Culture Collection of Algae and Protozoa, UK), Nannochloropsis oculata SAG38.85 and Microchloropsis salina SAG40.85 (Department Experimental Phycology and Culture Collection of Algae (EPSAG), Göttingen University, Germany.
The marine strains were cultivated in an artificial seawater (ASW) medium prepared based on Breuer et al. (2013a). Nutrients were supplied in excess; nitrogen and phosphorous were added to support a biomass concentration of 4 g L-1, assuming a 10% and 1% w/w biomass content of nitrogen and phosphorous, respectively. This was done to prevent nutrient limitations. In starvation conditions, there was no nitrogen in the medium, and only the amount of phosphate was adjusted to prevent any limitation.
The complete artificial seawater medium with modified Nutribloom per liter contained final concentrations of NaCl 418.14 mM; Na2SO4 13.33 mM; CaCl2·2H2O 5.42 mM; K2SO4 5.47 mM; MgCl2·6H2O 48.54 mM; NaNO3 42.82 mM; KH2PO4 1.03 mM; ZnSO4·7H2O 0.44 mM; MnCl2·4H2O 3.6 mM; Na2MoO4·2H2O 0.126 mM; CoCl2·6H2O 0.2 mM; CuSO4·5H2O 0.196 mM; Na2EDTA·2H2O 0.372 mM; MgSO4·7H2O 0.292 mM; FeCl3·6H2O 0.108 mM; and 20 mM HEPES (4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid). The pH of the medium was adjusted with 1 M NaOH to pH 7.5. For ASW (N-starvation), no NaNO3 was added.
Nannochloropsis limnetica was cultivated in BG-11 freshwater medium (Das and Kundu 2011), designed according to the chemical composition per liter with final concentration of Na2EDTA 0.269 mM; ferric ammonium citrate 0.16 mM; citric acid.1H2O 0.168 mM; CaCl2·2H2O 0.956 mM; MgSO4·7H2O; 0.986 mM; K2HPO4 0.646 mM; H3BO3 1.872 mM; MnCl2·4H2O 0.325 mM; ZnSO4·7H2O 0.030 mM; CuSO4·5H2O 0.024 mM; CoCl2·6H2O 0.017 mM; NaMoO4·2H2O 0.103 mM; Na2CO3 0.189 mM; NaNO3 17.65 mM, the pH of the growth medium was adjusted with 1M HCl to pH 7.5. For BG-11(N-starvation) no NaNO3 was added. The media were directly filtered and sterilized by Sartobran Capsule 0.2 μm into the Erlenmeyer flasks.
All strains were cultivated in duplicate with an initial optical density (OD) of 0.5 at 750 nm under identical controlled conditions in 250 mL Erlenmeyer flasks (150 mL of culture) in an orbital shaker incubator (Multitron, Infors HT, Switzerland) (100 rpm) with approximately 150 μmol photons m-2 s-1 continuous light supplied by fluorescent lamps (TL-D Reflex 36W/840, Philips) at 25 °C and 2% CO2 enriched air. The cultures were kept under a day/night cycle light regime (12:12 h).
Experimental approach
-
a.
The effect of light intensity
The duplicate flasks were pooled. Then, cells were resuspended in 50 mL Erlenmeyer flasks (25 mL of culture) of either N-replete or N-starvation medium at an OD750 of 0.5. The moment of resuspension was considered the beginning of the experiment (t=0). Finally, triplicate flasks were incubated for 12 days under identical controlled conditions in Algem HT24 from Algenuity on an orbital shaker incubator (Multitron, Infors HT, Switzerland) with 100 rpm at 25 °C and 2 % CO2 enriched air and two different light intensities of approximately 150 and 600 μmol photons m-2 s-1. The cultures were kept under a day/night cycle light regime (12:12 h).
-
b.
The effect of temperature
The duplicate flasks were pooled. Then, cells were resuspended in 50 mL Erlenmeyer flasks (25 mL of culture) of either N-replete or N-starvation medium at an OD750 of 0.5. The moment of resuspension was considered the beginning of the experiment (t=0). Finally, triplicate flasks were incubated for 12 days under identical controlled conditions in Algem HT24 from Algenuity on an orbital shaker incubator (Multitron, Infors HT, Switzerland) with 100 rpm at 150 μmol photons m-2 s-1 and 2 % CO2 enriched air and three different temperature degrees, approximately 15, 25, and 30 ℃. The cultures were kept under a sinus day/night cycle light regime (12:12 h).
Offline culture measurements
Samples were taken from all flasks at days 0, 2, 4, 6, 8, 10, and 12 for offline optical density (OD), cell concentration, and quantum yield (QY) measurements. Biomass concentration, dry weight (DW), and fatty acid analysis were measured at the experiments' beginning and end.
The optical density (OD) was measured at 750 nm using a UV-VIS spectrophotometer (Hack Lange DR-6000, light path 10 mm).
Cell concentration was measured with the mutlisizer III (Beckman Coulter) using a 50 μm aperture tube and after diluting the sample 100 times in ISOTON II diluent.
The photosystem II (PSII) maximum quantum yield (Fv/Fm) was measured after 20 min of dark adaptation at room temperature (AquaPen-C 100, PSI, Czech Republic; excitation light 455 nm, saturating light pulse: 3000 μmol photons m-2 s-1). The Fv/Fm ratio was calculated according to Benvenuti et al. (2016).
Biomass dry weight (Cx, g L-1) was measured from the moment of nitrogen starvation (t=0) as described by Breuer et al. (2013b).
Biomass volumetric production rate \({P}_{x}\) was calculated according to (Eq. (2)), where \({C}_{xt}\) and \({C}_{x0}\) correspond to the dry weight measured for day t and day 0, respectively, and T is the time of the experiment (12 days):
Nitrogen presence was monitored using the nitrate test (colorimetric, 10-500 mg L-1 (NO3-), for use with MQuant StripScan App). Although nitrogen was not directly measured, the nitrate levels served as an indicator of its presence. Samples were analyzed every 2 days to ensure consistent monitoring of nitrogen availability throughout the experiment.
Fatty acid analysis: The lipid composition of the different strains was measured at the experiment's beginning and end. Biomass samples were centrifuged, washed two times with 0.5M ammonium formate, and stored at -20 °C until lyophilization. Biomass fatty acid content (FFA, g g−1) and profile were determined by mechanical cell disruptions, solvent-based lipid extraction, and trans-esterification of fatty acids to fatty acid methyl esters (FAMEs). Lipids were extracted, separated into triacylglycerol (TAG) and polar (PL) fractions, and quantified with GC-FID as reported by Breuer et al. (2013a). Briefly, more than 10 mg (Tables 1S and 2S, show the exact amounts) of lyophilized biomass was disrupted with a beater. Lipids were extracted with chloroform:methanol (1:1.25, v:v) containing the internal standards for TAG and PL fractions, 170 μg mL−1 of tripentadecanoin (9:0) and 170 μg mL−1 of 1,2-dipentadecanoyl-sn-glycero-3-[phosphorrac-(1-glycerol)] (sodium salt) (15:0) respectively. Different elution solvents separated TAG and PL fatty acids, hexane:diethyl ether (7:1, v:v) and methanol:acetone:hexane (2:2:1, v:v), respectively, in a SPE silica gel column (Sep-Pak Vac 6cc, Waters), which were evaporated after that. Then, fatty acids were saponified and methylated in methanol with 5% H2SO4 at 100 °C for 1 h. The fatty acid methyl esters (FAMEs) were extracted with hexane and quantified on the GC-FID (Agilent 7890). The TAG and PL fatty acid contents were calculated by summing all fatty acids measured per mg of biomass in each lipid fraction.
where PFA= lipid productivity, LCt= final lipid content after 12 days of cultivation, Cxt= final biomass of the microalgae in the lipid-producing phase after 12 days of cultivation, LC0= initial lipid content at zero time, Cx0= initial biomass in the lipid-producing phase at zero time, T= the cultivation time.
Statistical analysis
Statistical analysis was performed using one-way ANOVA and two-way ANOVA followed by comparing means in cross-tabulation and the Bonferroni t-test (IBM SPSS Statistics 28 software)., All results are expressed as mean ± standard deviation (SD) (n=3) at a significance level of P< 0.05.
Results and discussion
Nannochloropsis and Microchloropsis are relevant in microalgal research and biotechnological applications due to their capability to produce high contents of triacylglycerols (TAGs) and essential omega-3 polyunsaturated acids like eicosapentaenoic (EPA Rodolfi et al. 2009; Benvenuti et al. 2014). Strains of Nannochloropsis and Microchloropsis can thrive in marine and freshwater (N. limnetica) habitats. This adaptability is critical for cultivating these algae in seawater, which does not compete with the freshwater resources needed for agriculture, increasing its sustainability (Converti et al. 2009; Simionato et al. 2013).
In this study, we systematically evaluated eight Nannochloropsis and Microchloropsis strains under varied abiotic stressors, including nitrogen availability, light intensity, and temperature. This comprehensive approach provides a systematic and robust comparative analysis showing not only species-specific responses but also strain-specific responses and new insights into optimizing microalgal lipid production.
The effect of the different light intensities and nitrogen availability on
-
a.
Photosynthetic efficiency.
When screening microalgae for lipid productivity it is essential to know the photosynthetic capacity under stress conditions. Maximum quantum yield (Fv/Fm) values around 0.6–0.7 are expected for healthy cells, whereas lower values are observed if cells have been exposed to biotic or abiotic stress (Young and Beardall 2003; Simionato et al. 2013). In our study Fv/Fm of cultures cultivated in nitrogen (N)-replete conditions did not change over time in low light (LL) (Fig. 1A). However, the quantum yield decreased in the first two days when the cells were exposed to high light (HL) and then increased to the typical values (Fig. 1C). This confirmed the findings of Benvenuti et al. (2014) where PSII maximum quantum yield is independent of growth irradiance as long as nutrients are not limiting and cells are fully photoacclimated.
On the contrary, N-starvation resulted in a significant decrease in quantum yield for all strains (Fig. 1, with a substantial decline in Fv/Fm by the end of the 12-day cultivation period in N-starved cultures under LL. Notably, cells grown in HL exhibited an even greater decrease than those in LL. The lowest Fv/Fm values were observed in N. oceanica IMET1 and Necton, at 0.23 and 0.28, respectively, while N. limnetica showed the highest Fv/Fm of 0.54 at HL. This may be attributed to the fact that, out of the eight strains studied, only N. limnetica was cultured in freshwater. The ability of freshwater N. limnetica to maintain its Fv/Fm under N-starvation likely results from its evolutionary adaptation to freshwater environments, involving efficient light harvesting, nutrient utilization and specialized physiological and metabolic mechanisms for coping with nutrient fluctuations, unlike the marine strains (Ma et al. 2014).
-
b.
Lipid content.
Figure 2 shows variations in the total lipid content (%) during the cultivation of different strains under varying light intensities and nitrogen availability conditions. Our findings reveal that all cells exhibited a modest increase in lipid content under high light intensities under N-repletion conditions. Notably, there were no substantial variations in lipid content among the different strains. Among all the species examined, M. gaditana CCFM01 displayed the highest lipid content, reaching 18.0% at HL (Fig. 2B).
Lipid content (mg mgDW-1%) in the triacylglycerol (TAG) and polar (PL) fractions of different Nannochloropsis and Microchloropsis species cultivated in nitrogen repletion (N+) (A) and nitrogen starvation (N-) (B) conditions under different light intensities 150 and 600 μmol photons m−2 s−1. Error bars represent the standard deviation of the mean (n=3)
N-starvation, regardless of light intensity, led to notable variations in lipid content among the different strains. There was a substantial boost in the total lipid content (%) across all strains under N-starvation, particularly at a light intensity of 600 μmol photons m−2 s−1 compared to 150 μmol photons m−2 s−1. For instance, N. sp exhibited the highest lipid content under LL conditions, reaching 44.9%, while N. oculata had the lowest lipid content (22.6%). HL-induced lipid accumulation for all strains, with N. sp. having a peak lipid concentration of 59.0%, 30% higher than in M. gaditana CCFM01, making N. sp. highly suitable for various applications where high lipid yield is essential. Various factors, including environmental stimuli, genetic heterogeneity, developmental stages, acclimation responses, and nutrient fluxes, influence lipid content in Nannochloropsis and Microchloropsis species. In our case the strains under investigation originated from different geographic locations and are experiencing diverse cultivation conditions, resulting in genetic variations (Wacker et al. 2016; Shi et al. 2017). This variation was not confined to inter-generic comparisons but was also evident within individual species, potentially explaining the observed significant lipid content differences among strains (Mishra et al. 2019).
-
c.
TAG and EPA contents.
There were no significant differences in the TAG content (expressed as %) between the strains under both light regimes when cultivated in the N-replete medium (Fig 3). Under N-repletion conditions (Fig. 3A), TAG content was not significantly different among the evaluated algal strains. Microchloropsis gaditana CCFM01 had a TAG content of 8.2% under HL. Contrastingly, N-starvation prompted a pronounced rise in TAG synthesis in all strains (Fig. 3B). However, the response to N-starvation significantly varied among strains, regardless of light intensity. It is essential to emphasize that increased light intensity stimulated the cells to accumulate more TAG. Under HL, N. sp. exhibited the maximal TAG concentration of 59.6%, a value substantially higher than the 31.5% in N. oculata. Furthermore, under LL conditions, N. sp. achieved a TAG content of 37.1%, eclipsing the lowest recorded value by N. oculata by a substantial margin of 61.7%. This increase in intracellular lipids when microalgae are subjected to high light intensity may be attributed to the oxidative stress induced by photocatalysts. Moreover, the observed differences in TAG accumulation among the strains under various conditions underscore the complex interplay between environmental factors, cellular responses, and intracellular lipid dynamics. Furthermore, the TAG content in different microalgal species increases with higher light intensity and nitrogen starvation, while membrane lipids decrease under these conditions (Pal et al. 2011; He et al. 2015; Nogueira et al. 2015; Shi et al. 2017). These findings underscore the pivotal role of light intensity in modulating lipid composition and productivity in various microalgae species, offering valuable insights for applications in biotechnology and biofuel production. Nonetheless, it is essential to note that this phenomenon is not consistent across all microalgae species, as only some species exhibit oleaginous characteristics similar to those in the studied strains. For instance, it has been reported that low-light conditions favoured TAG production in Rhodomonas sp. (Oostlander et al. 2020) and Phaeodactylum tricornutum (Remmers et al. 2017). This variability underscores the complexity of microalgal lipid metabolism, which is influenced by biomass cultivation and mode of cultivation.
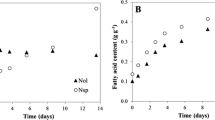
Cells can swiftly undergo ultrastructural modifications in response to changes in irradiance (Ma et al. 2016). In the present study EPA content (%) was negatively correlated with light intensity and nitrogen starvation. Under LL conditions (Fig. 4A.) all strains exhibited elevated levels of EPA with the highest value observed in N-replete M. gaditana CCFM01 cells (4.69%), followed by N. oculata (4.11%), which is significantly greater than the lowest value recorded by N. oceanica IMET1 (2.9%). This suggests an adaptive response to maximize light absorption and utilization under LL. Our findings indicate that M. gaditana CCFM01 and N. oculata cells are well-suited for nutraceutical applications where EPA is desired. These results are correlated with the quantum yield values, which were high at low light intensity. Higher quantum yield is associated with increased relative chloroplast volume, higher photosynthetic unit density, and a greater number of thylakoid stacks per chloroplast, which means more efficient photosynthesis and potentially higher EPA (Sukenik et al. 1989). Conversely, cells grown under higher light (Fig. 4B) displayed a marked reduction in EPA percentage, particularly under N-starvation. Microchloropsis salina had the lowest EPA content at 1.51%. In this scenario, the chloroplast volume decreased, leading to a concurrent reduction in EPA and increased neutral lipid oil droplets, TAG explicitly, within the cells (Sukenik et al. 1989). Our findings align with prior research, reinforcing that high light conditions or severe nitrogen limitation typically substantially reduce the proportion of polyunsaturated fatty acids, particularly EPA. This correlation has been documented in studies of Nannochloropsis sp. (Pal et al. 2011) and Phaeodactylum tricornutum (Mishra et al. 2019).
-
d.
Fatty acid profile.
EPA content (mg mgDW-1%) in the triacylglycerol (TAG) and polar (PL) fractions of different Nannochloropsis and Microchloropsis species cultivated in nitrogen repletion (N+) (A) and nitrogen starvation (N-) (B) conditions under different light intensities 150 and 600 μmol photons m−2 s−1. Error bars represent the standard deviation of the mean (n=3)
Figure 5 illustrates the impact of nitrogen availability and light conditions on the fatty acid profiles of the 8 strains examined in our study. Under N-repletion conditions (Fig. 5 A and C), the predominant fatty acids in all strains were C16:0 (palmitic acid), C16:1 (palmitoleic acid), C18:1 (oleic acid), and C20:5 (eicosapentaenoic acid), except for N. limnetica. This freshwater strain displayed a distinct fatty acid profile compared to the marine species, with C18:1 as the primary fatty acid and the absence of C20:5. In response to N-starvation, the proportions of these fatty acids exhibited a significant increase across the strains, except for EPA (C20:5), which displayed the opposite trend. Depending on the species, under low light (Fig. 5B) the marine strains demonstrated percentages ranging from 14% to 38% for C16:0 and 0.4% to 28% for C16:1 as a proportion of the total fatty acids when subjected to N-starvation. With higher light intensity (Fig. 5D), the C16 group represented the dominant percentages of the total fatty acids, ranging from 15 to 41% for C16:0 and 0.4 to 31% for C16:1. In the freshwater strain N. limnetica, under N-starvation and high light intensity, accumulated more than 50% of the total fatty acids as C18:1, with this proportion slightly declining when exposed to lower light intensity. These values are in accordance with several studies done on different microalgae species (Pal et al. 2011; Wagenen et al. 2012; Mishra et al. 2019; Conceição et al. 2020; Nzayisenga et al. 2020). It has been suggested that at a high light intensity, microalgal cells use excess energy to produce storage lipids, mainly SFA and MUFA, which are usually involved in the formation of thylakoid membranes. The decrease in PUFA, especially EPA, would be due to its role in synthesizing structural components (Wacker et al. 2016).
These observations underscore the significant influence of both nitrogen and light on the fatty acid composition of microalgae, emphasizing the intricate interplay between these factors and the resulting variations in fatty acid profiles.
-
e.
Biomass and lipid productivity.
Lipid productivity is a paramount factor in determining the economic viability of microalgae-based processes, as demonstrated in Tables 1 and 2. There was a positive correlation between increased light intensity and enhanced biomass productivity, with N. sp. and M. salina achieving the highest average biomass productivity, at 0.290 ± 0.016 and 0.222 ± 0.015 g L-1 day-1, respectively, under HL and N-replete conditions (Table 2). These findings align with Ma et al. (2014) who also found M. salina to have the highest biomass productivity among the nine tested strains, with a value of 0.363 ± 0.009 g L-1 day-1. Nevertheless, it is essential to note that biomass yield was approximately 50% lower in N-starved conditions than in N-replete media, irrespective of the light regime.
In addition to biomass productivity, we assessed lipid productivity in our study. Although the highest biomass productivity was observed under N-repletion, the highest lipid yields were consistently observed in N-starved cells, and this effect was notably accentuated under HL (Tables 1 and 2). There were statistically significant differences in lipids productivity between the strains, regardless of the conditions. The maximum daily average lipid productivity was achieved in N-starved cultures exposed to HL with N. sp., yielding 0.069 ± 0.004 g L-1 day-1. Microchloropsis salina closely followed with a lipid productivity of 0.062 ± 0.007 g L-1 day-1 (Table 2). Conversely, N. oculata exhibited the lowest lipid productivity under the same conditions, with 0.037± 0.007 g L-1 day-1. These variations in lipid productivity are primarily attributed to differences in biomass productivity between the strains since lipid productivity correlates strongly with biomass productivity. However, under N-starvation stress, although biomass productivity decreases, the lipid content increases as a survival mechanism, redirecting metabolic pathways to store energy in lipids. This shift can result in higher lipid productivity even with reduced biomass production, mainly if the increase in lipid content compensates for the drop in biomass (Huete-Ortega et al. 2018; Chin et al. 2023). These observations align with earlier research conducted on various Nannochloropsis and Microchloropsis species under light or nitrogen starvation stress, as well as in combination (Pal et al. 2011; Su et al. 2011; Wagenen et al. 2012; Fakhry and El Maghraby 2015). This detailed comparative data is crucial for selecting the robust strain for industrial lipid production.
Effect of temperature and nitrogen availability
Cultivation temperature is a critical parameter in microalgae cultivation. It influences various aspects of algal growth, as it affects chemical reaction rates, diffusion rates, membrane permeability, respiratory and photosynthetic electron transport, and enzyme activities, ultimately shaping the growth rate of algal populations. Furthermore, when selecting a microalgal strain for lipid production, it is essential to consider its lipid amount, fatty acid types, and biomass yield at different temperatures. Strains that grow well across various temperatures are beneficial, as they ensure steady production all year round, aiding in sustainable and dependable output.
In the second part of this study, we studied the effect of temperatures 15, 25, and 30℃ and nitrogen availability on biomass and lipid productivity at low light (LL) intensity 150 μmol photons m−2 s−1.
Photosynthetic efficiency
Temperature and nutrient availability significantly influence the microalgal metabolism, photosynthetic efficiency, growth, and biomass composition. The time-course variations of the photosynthetic activity (represented by Fv/Fm) at various temperatures are shown in Fig. 6 (A, B and C) under N-replete and Fig. 6 (D, E and F) under N-starvation conditions. The data reveal a consistent pattern in photosynthetic efficiency across different temperatures over 12 days under N-replete conditions, with minimal variations ranging from 0.63 to 0.73, depending on the species. This stability is noteworthy as it suggests the remarkable resilience of these species to temperature fluctuations, underlining their adaptability to a range of conditions (Fakhry and El Maghraby 2015; Ferrer-Ledo et al. 2023; Gao et al. 2023). However, N. oculata under N-replete conditionswas a notable exception a, where a sharp decrease in photosynthetic efficiency was evident, dropping to 0.26 at 30 ℃. This abrupt decline highlights the specific sensitivity of N. oculata to higher temperatures when nutrients are plentiful (Hung 2017).
Interestingly, the different species exhibited a slight decrease in photosynthetic efficiencies across varying temperatures under N-starvation conditions compared to N-replete conditions. Notably, N. oculata and N. limnetica displayed the lowest values at 30 and 15 ℃, with an efficiency of 0.25 for each. These results underline the dependence of microalgal photosynthetic efficiency on nutrient levels, and they can adjust to low nutrients but with less efficient photosynthesis (Ranglová et al. 2019; Peng et al. 2020; Ferrer-Ledo et al. 2023; Gao et al. 2023). Moreover, when grown at temperatures outside their ideal range, their photosynthesis suffers, likely due to enzyme issues. High temperatures affect enzymes crucial for growth, disrupt cell processes, and lower protein production, impairing photosynthesis, particularly the part that generates oxygen. This leads to less efficient energy transfer within the cells and increased stress, further reducing photosynthesis and overall cell health (He et al. 2018; Ferrer-Ledo et al. 2023).
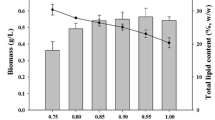
Lipid content
Temperature is a crucial environmental factor that needs thorough study because it dramatically affects the lipid makeup of photosynthetic organisms. Changes in temperature and lack of nitrogen impact the biomass, photosynthetic efficiency, and especially the lipid content in cells by altering chemical reaction rates and cell component stability (Sonmez et al. 2016). The effect of temperature on lipid content varies not only between different algal species but also among strains within the same species. The total lipid content of these strains varied significantly at different culture temperatures. In N-replete conditions (Fig. 7A) the various strains exhibited uniformity in lipid content across various temperatures with one notable exception. Nannochlorpsis oculata emerged as the outlier in this context, exhibiting the highest lipid content at 30 ℃, registering an impressive 31.8%, followed by N. sp. (22.3%), while N. limnetica had the lowest value (11.3%) at this temperature. Similarly, Wei et al. (2015); Hung (2017) found that in the presence of nitrogen, the highest lipid content in N . oculata was at 30°C, which was higher than its optimal growth temperature (25°C). Meanwhile, under N-starvation conditions (Fig. 7B), the lipid content doubled, depending on the strain, compared to N-repleted conditions. In this scenario, the decrease in the temperature from 25℃ to 15℃ led to an increase in the lipid content, and M. gaditana CCMP526 recorded a 45.6% lipid content at 15℃, which is twice as much as the lowest yielding strain, N. limnetica. These findings underscore the intricate relationship between temperature, nutrient availability, and strain-specific responses, illuminating the potential for tailored strategies to optimize lipid production in diverse microalgal species and strains. In addition, there is a wealth of literature documenting the impact of temperature on lipid accumulation in microalgae. Some reports have revealed a detrimental effect of temperature increases on various strains and their tendency to accumulate more lipids under low temperatures (e.g., Converti et al. 2009; Wagenen et al. 2012; Chaisutyakorn et al. 2018; Peng et al. 2020; Ferrer-Ledo et al. 2023). Conversely, other studies have demonstrated an increase in the lipid content of various Nannochloropsis species with rising temperatures, which is linked to the pivotal role of total lipid or increased reliance on lipids as a storage product (Fakhry and El Maghraby 2015; Malakootian et al. 2016). While various studies report different optimal temperatures for lipid accumulation in microalgae it is clear that the impact of temperature is complex and varies by species and even by strains in the same species. This variability could be due to the different temperature ranges used in these studies. Furthermore, the specific effects of temperature on lipid accumulation depend on the algal species or strain and their origin, with the exact mechanisms still unclear. However, it is generally observed that there is a negative correlation between lipid content and growth rate in microalgae under stress.
TAG and EPA contents
It has been observed in most microalgae that polar lipid content increases with decreasing temperature, whereas increased temperature leads to a higher accumulation of nonpolar lipids (TAG) (Renaud et al. 2002).
On the one hand, typically, when microalgae face stressful conditions, such as high irradiance, elevated temperatures and nitrogen deficiency, they tend to accumulate lipids, primarily in the form of TAG. Conversely, under ideal growth conditions, their lipid production remains relatively low (Wijffels and Barbosa 2010). Our findings demonstrate that under nitrogen repletion conditions Fig. 8A, all strains exhibited a similar trend in TAG accumulation, which was more favored at low temperatures, with the one notable exception of N. oculata. This strain had the highest TAG content of 17.6% at 30℃. This result can be attributed to the high stress experienced by N. oculata at the higher temperature, which in turn restricted its growth potential, ultimately resulting in elevated TAG levels.
In contrast, N. limnetica displayed the lowest TAG accumulation of only 2% at the same temperature, . In addition, under N-starvation (Fig. 8B) the TAG content in all strains doubled. However, the dynamics differed from those observed under N-repleted conditions. Surprisingly, 25℃ emerged as the optimal temperature for TAG accumulation in most strains, with N. sp and M. salina recording the highest contents of 36.9% and 35.3%, respectively, surpassing the lowest recorded value by N. oculata by 61%. Previous research on N. oceanica Necton has demonstrated it tends to accumulate higher levels of (TAG) in response to stress induced by low and high temperatures. This accumulation of TAG is believed to be part of a protective cellular mechanism aimed at safely dissipating excess carbon, energy, and electrons during unfavourable conditions. The exposure to low and high temperatures results in the build-up of reducing equivalents, which, if not effectively managed, can lead to the accumulation of reactive oxygen species. Thus, the synthesis of TAG serves as a vital complementary safety quenching mechanism (Ferrer-Ledo et al. 2023).
Nannochloropsis and Microchloropsis species hold significant industrial appeal due to their remarkable ability to accumulate high levels of eicosapentaenoic acid (EPA) compared to other microalgae-producing species. However, it is worth noting that the economic viability hinges on the current low productivity within photoautotrophic systems. Researchers have explored lowering temperatures as a strategic approach to elevate both EPA contents and productivity despite the potential growth impediments (Hoffmann et al. 2010). Figure 9A and B show the total EPA content and the distribution between polar and neutral lipids. The data reveal significant differences in total EPA accumulation among the various strains at different temperatures. Notably, it becomes evident that cells tend to accumulate more EPA outside the optimal growth temperature range.
EPA content (mg mgDW-1%) in the triacylglycerol (TAG) and polar (PL) fractions of different Nannochloropsis and Microchloropsis species cultivated under different temperatures 15, 25, and 30℃ cultivated in nitrogen repletion (N+) (A) and nitrogen starvation (N-) (B) conditions. Error bars represent the standard deviation of the mean (n=3)
Interestingly, the strains exhibit enhanced EPA accumulation at lower temperatures. Under N-repletion (Fig. 9A), at 15°C, N. oculata and M. gaditana CCMP526 significantly accumulated EPA across various temperatures, registering 5.7% and 5.1%, respectively. In contrast, M. gaditana CCFM01 accumulated 40% lower than N. oculata under the same conditions. These levels declined when the strains were subjected to nitrogen starvation (Fig. 9B); N. oculata achieved its peak EPA content of 5.7% at 15°C. In contrast, M. gaditana CCFM01 accumulated less than half the EPA amount of the highest recording strain under the same conditions. This decrease can be attributed to the reduction in EPA content in the polar fraction and the increase in the TAG fraction in response to nitrogen starvation stress. Moreover, the EPA content within lipid classes displayed temperature-dependent variations, suggesting a dynamic mobilization of EPA and a precise regulation of its synthesis and degradation processes (Ferrer-Ledo et al. 2023). Furthermore, it is noteworthy that the freshwater strain N. limnetica, under various cultivation conditions, did not exhibit significant EPA accumulation. Consistent with our findings, numerous studies have explored the impact of temperature on EPA content across various species of Nannochloropsis and Microchloropsis. These studies have consistently demonstrated a notable rise in EPA content in response to lower temperatures (Hoffmann et al. 2010; Mitra et al. 2015; Wei et al. 2015; Aussant et al. 2018; Willette et al. 2018; Sá et al. 2020).
Fatty acid profile
As mentioned earlier, the fatty acid composition is distinctly influenced by temperature fluctuations and this influence varies from strain to strain. Among all the strains under nitrogen repletion conditions (Fig. 7S (A, B, and C)) the prevailing fatty acids were C:14, C:16:0, C16:1 and C20:5. However, there was a significant change when the strains were exposed to nitrogen starvation (Fig. 7S (D, E, and F)). Under N-starvation, there was substantial increase in C:14, C:16:0, C16:1, and C18:0 in all strains except for N. limnetica, which featured C16:0 and C18:0 as the primary fatty acids. This shift can be attributed to the culture's adaptation under N-starvation, where the content of saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) significantly rises while polyunsaturated fatty acids (PUFAs) decrease (Fig. 8SB). Additionally, under these conditions, the composition of the fatty acids also varied with temperature fluctuations (Fig. 7S). Notably, most strains exhibited a decrease in the amounts of C16:1, C18:1, and C20:5, accompanied by an increase in C16:0 and C18:0 at 30°C. This may be because higher temperatures (25°C and 30°C) favour SFA synthesis, potentially as a mechanism to maintain cell membrane integrity under thermal stress. In contrast, lower temperatures (15°C) promote the synthesis of MUFAs and PUFAs, which are essential for membrane fluidity and function under cooler conditions (Fig. 8SB). The strain-specific responses to temperature changes underline the importance of selecting appropriate strains for specific environmental conditions to maximize lipid productivity.
While the impact of temperature on lipid content displays significant variation, the effects of temperature on the fatty acid profiles of microalgae exhibit remarkable consistency and have been extensively studied. Temperature-induced changes in FA composition play a pivotal role in modulating the physical properties of membranes, thereby facilitating the maintenance of essential metabolic functions under different temperatures, including photosynthesis, ion permeability, and respiration (Sheng et al. 2011; Peng et al. 2020; Gao et al. 2023).
In accordance with our results, numerous findings have consistently revealed that microalgae tend to increase their relative concentration of unsaturated FAs when exposed to lower temperatures (Aussant et al. 2018; Ferrer-Ledo et al. 2023). This response is attributed to the need for maintaining membrane fluidity, particularly thylakoid membranes, protecting the photosynthetic machinery from photoinhibition and ensuring the functionality of critical cellular processes (Chaisutyakorn et al. 2018). Conversely, at elevated temperatures, there is a propensity for increased saturated fatty acid (SFA) content, a strategy employed to preserve the integrity of microalgal cell membranes (Converti et al. 2009; Wagenen et al. 2012; Wei et al. 2015). The knowledge of the effect of temperature on fatty acid profiles can guide the targeted production of specific fatty acids, benefiting the biofuel and nutraceutical industries.
Biomass and lipid productivity
Among the foremost challenges in commercial microalgae utilization, two pivotal issues stand out: optimizing cell concentrations and enhancing the desired components' productivity. Tables 3 and 4 present data on biomass and lipid productivity of different Nannochloropsis and Microchloropsis species cultivated at different temperatures under N-repletion (Table 3) and N-starvation (Table 4). The data indicate a notable variance in the impact of temperature on biomass and lipid productivity across different strains. A key observation is that, for most strains 15°C was the optimal temperature for maximizing biomass and lipid productivity. Under N-repletion, among the different strains, M. salina stood out, exhibiting the highest biomass productivity at 15°C across all tested temperatures (0.156 g L-1 day-1), outperforming M. gaditana CCFM01, which recorded the lowest rate (0.079 g L-1 day-1). These results contradict the cell concentration data (Fig. 4S where at 25°C under N-repletion the highest cell concentration was reached by N. oceanica Necton and M. salina with 338.67 and 286.76 ×106 cells mL-1, respectively. This can be attributed to the differences in cell size between strains or different genera under specific conditions influencing biomass yields. For instance, at 15°C, slower metabolic rates might result in larger cell sizes as the duration of cell growth phases extends before division occurs. This can increase biomass per cell, enhancing overall biomass productivity if the cells accumulate more internal components (like lipids, carbohydrates, and proteins) before dividing. At 25°C, the higher temperature may accelerate metabolic processes and cell division rates without necessarily increasing the size of each cell (Huerlimann et al. 2010) resulting in higher cell concentrations, as cells divide more frequently. However, each cell may be smaller in size or less dense in biomass content, leading to lower overall biomass productivity compared to the scenario at 15°C. In addition, biomass productivity, typically measured as the dry weight per volume of culture per unit time, is a function of cell size and number (Borowitzka 2016). Larger, fewer cells might yield higher biomass productivity due to greater internal component accumulation. In contrast, cultures with smaller, more numerous cells might show elevated cell concentrations with proportionately less biomass per cell (Renaud et al. 2002).
In terms of lipid productivity, both M. salina and M. gaditana CCMP526 achieved peak lipid productivity at 15°C under N-starvation, with rates of 0.069 and 0.063 g L-1 day-1, respectively surpassing M. gaditana CCFM01 by approximately 70%. This suggests that M. salina could be optimal for cooler climate operations focusing on lipid production. Our findings align with numerous studies highlighting the impact of temperature on lipid accumulation in microalgae. For instance, in the case of different species of Nannochloropsis, the peak fatty acid productivity was observed at low temperatures, underscoring the influential role of temperature (Hoffmann et al. 2010; Malakootian et al. 2016; Chaisutyakorn et al. 2018). However, it is noteworthy that our results contrast with the commonly observed pattern, as they observed that low temperatures had a relatively minor impact, whereas high temperatures maximized lipid production (Ho et al. 2014; Han et al. 2016; Peng et al. 2020), thus providing new insights into the temperature-lipid productivity relationship.
The study's novelty lies in its comparative approach, which reveals significant genus and strain-specific response differences and the interactions of multiple environmental stressors such as light intensity, temperature, and nitrogen availability. The research identifies optimal growth and lipid production conditions for specific strains, demonstrating notable variability even within the same genus. These findings highlight the importance of considering multiple stressors simultaneously to develop effective strategies for maximizing lipid yield in microalgae. The knowledge gained can drive advancements in microalgal biotechnology, leading to more cost-effective and sustainable production methods.
Conclusion
This comprehensive study systematically evaluated eight oleaginous microalgal strains of Nannochloropsis and Microchloropsis for their growth and lipid accumulation abilities under various abiotic stressors, including nitrogen limitation and light and temperature variations, contributing significantly to existing knowledge. The results demonstrated significant variations in response to these environmental conditions among the strains. A substantial increase in lipid production was noted under HL and N-starvation conditions, albeit with considerable variability across different strains. Notably, N. sp. emerged as the top performer in lipid productivity (0.069 g L-1 day-1), while N. oculata recorded the lowest (0.037 g L-1 day-1). In addition, under N-repletion at LL conditions, M. gaditana CCFM01 exhibited the highest EPA content (4.7%), contrasting with N. oceanica IMET1’s lowest EPA content (2.9%).
The study also revealed that the optimal temperature for growth and lipid production and the response to temperature varied significantly between species, not only from different genera but also within the same genus and even among different strains of the same species, likely due to differences in their native environments. Among the strains tested, the highest lipid productivity was achieved by M. salina, registering 0.069 g L-1 day-1, surpassing M. gaditana CCFM01 by approximately 70% at 15℃ under N-starvation conditions. Moreover, N-starvation and temperature fluctuations markedly influenced the composition of fatty acids. SFAs and MUFAs significantly rose, while PUFAs in response to N-starvation. In addition, as the culture temperature decreased, SFAs significantly reduced, while the content of MUFAs and PUFAs, primarily EPA, increased. In this regard, N. oculata had the highest EPA accumulation, reaching 5.7% at 15 ℃ under N-repletion, which is substantially higher by 40% compared to M. gaditana CCFM01.
Despite the study limitations, including the monitoring of nitrogen presence, and the study's limited duration of 12 days, this study underscores the potential of stressors as promising tools in algal photobiotechnology, enabling the production of specific lipid classes and emphasizing the significance of acquiring knowledge the physiology and responses of various species, even within the same genus. This knowledge is a clear guide for selecting the robust strain with optimal operation conditions for specific applications, which is critical for developing efficient and sustainable microalgae-based industrial lipid production.
Future research can build on our findings by exploring the genetic and metabolic pathways responsible for the observed strain-specific responses using omics technologies. Additionally, longer-term studies are needed to assess the sustainability of lipid production in continuous or semi-continuous systems. Addressing the economic and practical aspects of scaling up these processes through techno-economic analyses and pilot-scale experiments will also be crucial for developing an efficient and sustainable microalgal lipid-production industry.
Data availability
The raw data supporting the findings of this study will be available by the authors.
References
Abdel-Karim OH, Gheda SF, Ismail GA, Abo-Shady AM (2020) Phytochemical Screening and antioxidant activity of Chlorella vulgaris. Delta J Sci 41:81–91
Abo-Shady AM, Gheda SF, Ismail GA, Cotas J, Pereira L, Abdel-Karim OH (2023) Antioxidant and antidiabetic activity of algae. Life 13:1–26
Aratboni HA, Rafiei N, García-Granados R, Alemzadeh A, Morones-Ramírez JR (2019) Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact 18:1–17
Aussant J, Guihéneuf F, Stengel DB (2018) Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl Microbiol Biotechnol 102:5279–5297
Barati B, Gan SY, Lim PE, Beardall J, Phang SM (2019) Green algal molecular responses to temperature stress. Acta Physiologiae Plantarum 41:1–19
Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2014) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27:1425–1431
Benvenuti G, Bosma R, Ji F, Lamers P, Barbosa MJ, Wijffels RH (2016) Batch and semi-continuous microalgal TAG production in lab-scale and outdoor photobioreactors. J Appl Phycol 28:3167–3177
Borowitzka M (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The Physiology of Microalgae. Springer, Cham, 6:601–652
Breuer G, Evers WA, de Vree JH, Kleinegris DM, Martens DE, Wijffels RH, Lamers PP (2013a) Analysis of fatty acid content and composition in microalgae. J Visualiz Exp 80:1–8
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2013b) Effect of light intensity, pH, and temperature on triacylglycerol (TAG) accumulation induced by nitrogen starvation in Scenedesmus obliquus. Bioresour Technol 143:1–9
Chaisutyakorn P, Praiboon J, Kaewsuralikhit C (2018) The effect of temperature on growth and lipid and fatty acid composition on marine microalgae used for biodiesel production. J Appl Phycol 30:37–45
Chin GJWL, Andrew AR, Abdul-Sani ER, Yong WTL, Misson M, Anton A (2023) The effects of light intensity and nitrogen concentration to enhance lipid production in four tropical microalgae. Biocatal Ag Biotechnol 48:1-12
Chisti Y (2007) Biodiesel from microalgae. Biotech Adv 25:294–306
Conceição D, Lopes RG, Derner RB, Cella H, do Carmo APB, Montes D’Oca MG, Petersen R, Passos MF, Vargas JVC, Galli-Terasawa LV, Kava V (2020) The effect of light intensity on the production and accumulation of pigments and fatty acids in Phaeodactylum tricornutum. J Appl Phycol 32:1017–1025
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process: Process Intensif 48:1146–1151
Das D, Kundu M (2011) Identification of algal biomass production with partial least squares & neural network. Int J Chem Eng Appl 2:288–293
El Shafay SM, Gaber A, Alsanie WF, Elshobary ME (2021) Influence of nutrient manipulation on growth and biochemical constituent in Anabaena variabilis and Nostoc muscorum to enhance biodiesel production. Sustainability 13:9081
Elshobary ME, Zabed HM, Qi X, El-Shenody RA (2022) Enhancing biomass and lipid productivity of a green microalga Parachlorella kessleri for biodiesel production using rapid mutation of atmospheric and room temperature plasma. Biotech Biofuels Bioprod 15:1–17
Fakhry EM, El Maghraby DM (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Bot Stud 56:1–8
Fawley KP, Eliáš M, Fawley MW (2014) The diversity and phylogeny of the commercially important algal class Eustigmatophyceae, including the new clade Goniochloridales. J Appl Phycol 26:1773–1782
Ferrer-Ledo N, Stegemüller L, Janssen M, Wijffels RH, Barbosa MJ (2023) Growth and fatty acid distribution over lipid classes in Nannochloropsis oceanica acclimated to different temperatures. Front Plant Sci 14:1–16
Gaignard C, Zissis G, Buso D (2021) Influence of different abiotic factors on lipid production by microalgae – a review. Oilseeds Fats Crops Lipids 57:1–16
Gao B, Hong J, Chen J, Zhang H, Hu R, Zhang C (2023) The growth, lipid accumulation and adaptation mechanism in response to variation of temperature and nitrogen supply in psychrotrophic filamentous microalga Xanthonema hormidioides (Xanthophyceae). Biotech Biofuels Bioprod 16:1–16
Gheda SF, Abo-Shady AM, Abdel-Karim OH, Ismail GA (2021) Antioxidant and antihyperglycemic activity of Arthrospira platensis (Spirulina platensis) methanolic extract: in vitro and in vivo Study. Egypt J Bot 61:71–93
Gui J, Chen S, Luo G, Wu Z, Fan Y, Yao L, Xu H (2021) Nutrient deficiency and an algicidal bacterium improved the Lipid profiles of a novel promising oleaginous dinoflagellate, Prorocentrum donghaiense, for biodiesel production. Appl Environ Microbiol 87:1–17
Han F, Pei H, Hu W, Han L, Zhang S, Ma G (2016) Effect of high-temperature stress on microalgae at the end of the logarithmic phase for the efficient production of lipid. Environ Technol 37:2649–2657
Hanifzadeh M, Nabati Z (2019) Production of biodiesel from microalgae. World biodiesel policies and production, 1st edn. CRC Press, Boca Raton, pp 1–34
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
He Q, Yang H, Hu C (2018) Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour Technol 265:407–414
Ho S-H, Chang J-S, Lai Y-Y, Chen C-NN (2014) Achieving high lipid productivity of a thermotolerant microalga Desmodesmus sp. F2 by optimizing environmental factors and nutrient conditions. Bioresour Technol 156:108–116
Hoffmann M, Marxen K, Schulz R, Vanselow KH (2010) TFA and EPA Productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar Drugs 8:2526–2545
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257
Huete-Ortega M, Okurowska K, Kapoore RV, Johnson MP, Gilmour DJ, Vaidyanathan S (2018) Effect of ammonium and high light intensity on the accumulation of lipids in Nannochloropsis oceanica (CCAP 849/10) and Phaeodactylum tricornutum (CCAP 1055/1). Biotechnol Biofuels 11:1–15
Hulatt CJ, Smolina I, Dowle A, Kopp M, Vasanth GK, Hoarau GG, Wijffels RH, Kiron V (2020) Proteomic and transcriptomic patterns during lipid remodeling in Nannochloropsis gaditana. Int J Mol Sci 21:1–23
Hung NT (2017) Effect of temperature, light on the growth and lipid accumulation of Nannochloropsis oculata. Int J Appl Environ Sci 12:449–445
Ippoliti D, González A, Martin Cara I, Fernandez-Sevilla JM, Pistocchi R, Acien G (2016) Outdoor production of Tisochrysis lutea in pilot-scale tubular photobioreactors. J Appl Phycol 28:3159–3166
Jia J, Han D, Gerken HG, Li Y, Sommerfeld M, Hu Q, Xu J (2015) Molecular mechanisms for photosynthetic carbon partitioning into storage neutral lipids in Nannochloropsis oceanica under nitrogen-depletion conditions. Algal Res 7:66–77
Juergens MT, Deshpande RR, Lucker BF, Park J-J, Wang H, Gargouri M, Holguin FO, Disbrow B, Schaub T, Skepper JN, Kramer DM, Gang DR, Hicks LM, Shachar-Hill Y (2015) The regulation of photosynthetic structure and function during nitrogen deprivation in Chlamydomonas reinhardtii. Plant Physiol 167:558–573
Karima A, Silalahi MDS, Rinanti A (2018) Increasing content of lipid in tropical microalgae Chlorella sorokiniana and Closterium sp. with variation of nitrogen content and extraction temperature. MATEC Web Conf 197:1–4
Klok AJ, Martens DE, Wijffels RH, Lamers PP (2013) Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243
Li-Beisson Y, Beisson F, Riekhof W (2015) Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J 82:504–522
Ma Y, Wang Z, Yu C, Yin Y, Zhou G (2014) Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour Technol 167:503–509
Ma XN, Chen TP, Yang B, Liu J, Chen F (2016) Lipid production from Nannochloropsis. Mar Drugs 14:1–18
Malakootian M, Hatami B, Dowlatshahi S, Rajabizadeh A (2016) Growth and lipid accumulation in response to different cultivation temperatures in Nannochloropsis oculata for biodiesel production. Env Health Eng Manage J 3:29–34
Maltsev Y, Maltseva K, Kulikovskiy M, Maltseva S (2021) Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 10:1–24
Maltsev Y, Kulikovskiy M, Maltseva S (2023) Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae. Microb Cell Fact 22:1–22
Metsoviti MN, Papapolymerou G, Karapanagiotidis IT, Katsoulas N (2020) Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 9:1–17
Mishra N, Prasad SM, Mishra N (2019) Influence of high light intensity and nitrate deprivation on growth and biochemical composition of the marine microalgae Isochrysis galbana. Braz Arch Biol Technol 62:1–13
Mitra M, Patidar SK, George B, Shah F, Mishra S (2015) A euryhaline Nannochloropsis gaditana with potential for nutraceutical (EPA) and biodiesel production. Algal Res 8:161–167
Morales M, Aflalo C, Bernard O (2021) Microalgal lipids: a review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 150:1–25
Naduthodi MIS, Sudfeld C, Avitzigiannis EK, Trevisan N, van Lith E, Alcaide Sancho J, D’Adamo S, Barbosa M, van der Oost J (2021) Comprehensive genome engineering toolbox for microalgae Nannochloropsis oceanica based on CRISPR-Cas Systems. ACS Synth Biol 10:3369–3378
Nadzir SM, Yusof N, Nordin N, Abdullah H, Kamari A (2018) Combination effect of temperature and light Intensity on lipid productivity of Tetradesmus obliquus. J Phys Conf Ser 1097:1–10
Nogueira KDP, Silva AF, Araújo OQF, Chaloub RM (2015) Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass Bioenergy 72:280–287
Nzayisenga JC, Farge X, Groll SL, Sellstedt A (2020) Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol Biofuels 13:1–8
Oostlander PC, van Houcke J, Wijffels RH, Barbosa MJ (2020) Optimization of Rhodomonas sp. under continuous cultivation for industrial applications in aquaculture. Algal Res 47:1–9
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Park H, Lee C-G (2016) Theoretical calculations on the feasibility of microalgal biofuels: utilization of marine resources could help realizing the potential of microalgae. Biotechnol J 11:1461–1470
Peng X, Meng F, Wang Y, Yi X, Cui H (2020) Effect of pH, temperature, and CO2 concentration on growth and lipid accumulation of Nannochloropsis sp. MASCC 11. J Ocean Univ China 19:1183–1192
Ranglová K, Lakatos GE, Manoel JC, Grivalský T, Masojídek J (2019) Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol 64:615–625
Remmers IM, Martens DE, Wijffels RH, Lamers PP (2017) Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities. PLoS One 12:1–13
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Ryu AJ, Kang NK, Jeon S, Hur DH, Lee EM, Lee DY, Jeong BR, Chang YK, Jeong KJ (2020) Development and characterization of a Nannochloropsis mutant with simultaneously enhanced growth and lipid production. Biotechnol Biofuels 13:1–14
Sá M, Ferrer-Ledo N, Wijffels R, Crespo JG, Barbosa M, Galinha CF (2020) Monitoring of eicosapentaenoic acid (EPA) production in the microalgae Nannochloropsis oceanica. Algal Res 45:1–10
Schulze PSC, Hulatt CJ, Morales-Sánchez D, Wijffels RH, Kiron V (2019) Fatty acids and proteins from marine cold adapted microalgae for biotechnology. Algal Res 42:1–9
Sheng J, Kim HW, Badalamenti JP, Zhou C, Sridharakrishnan S, Krajmalnik-Brown R, Rittmann BE, Vannela R (2011) Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour Technol 102:11218–11225
Shi K, Gao Z, Shi T-Q, Song P, Ren L-J, Huang H, Ji X-J (2017) Reactive oxygen species-mediated cellular stress response and lipid accumulation in oleaginous microorganisms: the state of the art and future perspectives. Front Microbiol 8:1–9
Sibi G, Shetty V, Mokashi K (2016) Enhanced lipid productivity approaches in microalgae as an alternate for fossil fuels – A review. J Energy Inst 89:330–334
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676
Sivagurulingam AP, Sivanandi P, Pandian S (2022) Isolation, mass cultivation, and biodiesel production potential of marine microalgae identified from Bay of Bengal. Env Sci Pollut Res 9:6646–6655
Solovchenko A, Lukyanov A, Solovchenko O, Didi-Cohen S, Boussiba S, Khozin-Goldberg I (2014) Interactive effects of salinity, high light, and nitrogen starvation on fatty acid and carotenoid profiles in Nannochloropsis oceanica CCALA 804. Eur J Lipid Sci Technol 116:635–644
Sonmez C, Elcin E, Akın D, Oktem HA, Yucel M (2016) Evaluation of novel thermo-resistant Micractinium and Scenedesmus sp. for efficient biomass and lipid production under different temperature and nutrient regimes. Bioresour Technol 211:422–428
Su C-H, Chien L-J, Gomes J, Lin Y-S, Yu Y-K, Liou J-S, Syu R-J (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sukenik A, Carmeli Y, Berner T (1989) Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol 25:686–692
Wacker A, Piepho M, Harwood JL, Guschina IA, Arts MT (2016) Light-induced changes in fatty acid profiles of specific lipid classes in several freshwater phytoplankton species. Front Plant Sci 7:1–13
Wagenen VJ, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann M (2012) Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5:731–740
Wei L, Huang X, Huang Z (2015) Temperature effects on lipid properties of microalgae Tetraselmis subcordiformis and Nannochloropsis oculata as biofuel resources. Chin J Oceanol Limnol 33:99–106
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Willette S, Gill SS, Dungan B, Schaub TM, Jarvis JM, St. Hilaire R, Omar Holguin F (2018) Alterations in lipidome and metabolome profiles of Nannochloropsis salina in response to reduced culture temperature during sinusoidal temperature and light. Algal Res 32:79–92
Young E, Beardall J (2003) Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J Phycol 39:897–905
Funding
This project has received funding by a full scholarship (2020/2021) from the Ministry of Higher Education & Scientific Research, Egypt.
Author information
Authors and Affiliations
Contributions
Omnia H. Abdelkarim, Maria J. Barbosa, and Rene H. Wijffels conceived the research and designed the experiments. OHA performed the experiments and analysed and interpreted the data. OHA wrote the original draft. RHW and MJB supervised and edited the manuscript. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that this research was conducted without any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelkarim, O.H., Wijffels, R.H. & Barbosa, M.J. Microalgal lipid production: A comparative analysis of Nannochloropsis and Microchloropsis strains. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03318-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03318-7