Abstract
Cultivation of microalgae has gained significant interest as an alternative protein source, potentially becoming a target commodity recovered from microalgae-based wastewater treatment. This study examined a semi-continuous cultivation strategy to optimize protein accumulation of the indigenous freshwater chlorophytes, Lobochlamys segnis and Klebsormidium flaccidum, and simultaneously remove nutrients from wastewater efficiently. A strain-specific regime was made based on a fixed biomass concentration at the start of 24-h cultivation cycle, i.e., a constant initial cell density, which regulated harvesting and fresh medium supply volume according to the dilution rate. Six cultivation cycles were conducted in lab-scale 1L reactors with a synthetic municipal wastewater. Lobochlamys segnis and K. flaccidum grew exponentially in all cycles. The biomass productivity was 573 and 580 mg L–1 day–1, in which the total protein consisted of 62 and 45% of dry cell weight (dw), respectively. When a culture medium deficient in nitrogen and phosphorus was used, protein level was significantly reduced. L. segnis consumed all NH4+ and PO43– supplied by the medium replacement, giving the removal rate of 9.2 and 5.2 mg L–1 day–1. Whereas K. flaccidum removed 13.8 mg L–1 day–1 NH4+ without completing PO43– removal. The amino acid profile of both strains was characterized by glutamic acids content (4–5% dw). We concluded that the designed cultivation regime would support a constant biomass production with stable and high protein content, along with an efficient removal of nutrient from the wastewater.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Microalgae proteins have a great potential to contribute to the recent increasing demand for protein source (Janssen et al. 2022). Commercially produced microalgae generally contain a high proportion of proteins, e.g. Arthrospira platensis 70% (Avila-Leon et al. 2012), Tetraselmis sp. 64% (Schwenzfeier et al. 2011), and Chlorella vulgaris 44% (Seyfabadi et al. 2011), with whole cells of these species already processed as food and feed supplements (Ritala et al. 2017). However, high capital investment and production cost of microalgae protein cultivation has constrained commercial interest, potentially obstructing their market relevance for low-priced commodity production, for example for pet/livestock feeds (Geada et al. 2021). Recent increased attention in alternative protein source has refocused in microalgae protein (Janssen et al. 2022), as their cultivation is suggested to be superior to land crops for the output quality, environmental impact, productivity per land area and water requirements (Fernández et al. 2021). In fact, autotrophic microalgae are proposed as an advantageous choice of microorganisms for the single-cell protein industry in terms of environmental costs and benefits, because of their CO2 capture and storage capability (Geada et al. 2021).
A promising way to strengthen the economic viability of microalgae cultivation is to combine the protein production with the wastewater treatment, where nutrients, water and dissolved inorganic carbon in the waste streams can be exploited for the microalgae biomass generation (Nagarajan et al. 2020). For example, nutrient requirements are reported as > 70% of the total cost for microalgae production in an open raceway system (Acién et al. 2014), indicating the potential of nutrient recycling from wastewater for reducing operation costs (Fernández et al. 2021). An ideal approach is an integrating microalgae culture and protein manufacturing into a circular bioeconomy system (Fuentes-Grünewald et al. 2021), where the side streams (production-, process- and wastewater) of agriculture or aquaculture provide readily available nutrients for microalgae, while the generated biomass is converted into a protein source for animal feeds. This can be realized if the system supports sufficient microalgal biomass production and that the produced microalgae contain sufficient quantity and quality of protein.
Protein is a crucial ingredient for animal feeds, providing a source of nitrogen and essential amino acids. Microorganisms have long been used for animal feed manufacturing, especially certain bacteria which can typically contain 50-80% protein (Ritala et al. 2017). Despite intra- and interspecific variation in protein content (Muys et al. 2019), microalgae can contain similar protein amounts compared to bacteria, and these proteins are assessed as suitable for animal feeds by nutritional and toxicological evaluations (Becker 2007). The nutritional value of the protein is determined by the quantity, balance and bioavailability of amino acids (Wu 2014). In addition to essential amino acids (EAAs) (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine), other amino acids sufficiently synthesizable by animals (non-essential amino acids) are also indispensable in animal diets (Wu and Li 2022). To understand the nutritional potential of microalgae species for protein production, it is therefore, essential to profile their amino acids composition under production conditions.
A development strategy for microalgae production of desired protein properties in the microalgae-based wastewater treatment systems is strategic cultivation of target strains, where desired microalgae protein accumulation can be achieved by applying a suitable nutrient regime. Such a strategy requires less advanced technology and avoids legislation problems in comparison to approaches involving genetic metabolic engineering (Procházková et al. 2014). Recent studies have shown that microalgae can assimilate nutrients from urban (Oss et al. 2022) and aquaculture wastewater (Akmukhanova et al. 2022), as well as liquid digestate from anaerobic digestion (Seelam et al. 2022). Nevertheless, challenges remain to developing marketable products from the biomass generated from microalgae-based wastewater treatment (Fernández et al. 2021). Utilization of wastewater for industrial microalgae production targeted to human consumption or animal feeds is restricted by current legislations for safety concern (Fernández et al. 2021; Show 2022). Thus, there are many technical, production and safety challenges to overcome towards combining microalgae protein production and wastewater treatment. However, of primary importance is demonstrating viability of systems using microalgae species/strains to produce a desired protein quality in sufficient quantity, while treating wastewater with a desired efficacy.
In this study we proposed a semi-continuous cultivation regime using wastewater to enhance microalgae protein production. Previously, we found that the protein contents in selected indigenous chlorophyte strains were the highest in the early stage of the batch cultivation when they had the highest growth rate (Umetani et al. 2023). The protein share in their biomass was reduced significantly at the time when nitrogen was depleted from their culture medium. These findings suggest that the high protein contents of the strains may be maintained if microalgae growth is kept at an exponential phase, and if there is sufficient available nitrogen. Therefore, this study 1) established a regime for semi-continuous mode cultivation for a regulation of cell density and nutrient supply to sustain exponential growth, 2) tested whether the protein content of the selected microalgae can be maintained under the semi-continuous cultivation regime, 3) evaluated if the regime was effective for nutrient removal from wastewater. The main aim of this study was to develop a suitable cultivation method for microalgae protein production with a maximal usage of nutrients recycled from wastewater. Although further studies in scale-up scenarios using real wastewater are imperative, this study provides a fundamental cultivation strategy to enhance protein accumulation in microalgae grown in wastewater.
Materials & methods
Microalgae and cultivation condition
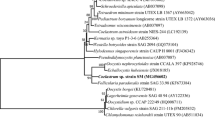
Two freshwater algae, Lobochlamys segnis F12 (Chlorophyta, Chlamydomonadales) and Klebsormidium flaccidum NIVA-CHL80 (Charophyta, Klebsormidiales) were chosen as model organisms. Lobochlamys segnis F12 (Fig. 1a) was isolated and maintained as a mono-species culture using Bolds Basal Medium (Bischoff and Bold 1963). Species identification of L. segnis F12 was based on an 18S rDNA phylogeny (Online Resource Fig. 1; GenBank accession MT973497) and morphology (Pröschold et al. 2001). Klebsormidium flaccidum NIVA-CHL80 (Fig. 1b) was obtained from the Norwegian Culture Collection of Algae (NORCCA), and the stock culture was maintained using the Z8 medium (Kotai 1972). We selected these strains based on the results of our previous work, where L. segnis F12 achieved efficient nutrient (nitrogen and phosphorus) removal, and K. flaccidum NIVA-CHL80 contained the highest protein level among the studied strains (Umetani et al. 2023).
The medium used for inoculum and experiment cultures was a synthetic municipal wastewater prepared based on the previous studies (Tiron et al. 2015, 2017; Umetani et al. 2023). It contained NH4Cl: 178 mg L–1, K2HPO4: 56 mg L–1, CaCl2: 40 mg L–1, MgSO4·7H2O: 75 mg L–1, FeSO4·7H2O: 5 mg L–1, Na2EDTA·2H2O: 2.5 mg L–1, H3BO3: 50 μg L–1, ZnCl2: 50 μg L–1, CuSO4·5H2O: 30 μg L–1, MnCl2·4H2O: 39 μg L–1, (NH4)6Mo7O24·4H2O: 50 μg L–1, KAl(SO4)2: 178 μg L–1, CoCl2·6H20: 50 μg L–1, NiSO4·6H2O: 101 μg L–1, sodium acetate: 200 mg L–1 and NaHCO3: 750 mg L–1. All medium components were dissolved in dH2O and autoclaved, except NaHCO3, which was filter-sterilized and added to the autoclaved medium.
The experiment cultures were grown in 300 mL medium in 1-L Erlenmeyer flask under continuous illumination providing 70 μmol photons m–2 s–1 irradiance in the incubator at 24 °C (Infors HT Multitron Pro, infors AG, Switzerland). The light and temperature condition were selected based on a previous study of the microalgae strains (Umetani et al. 2023). The cultures received aeration (2 L min–1) with filtered (pore size of 0.2 μm) air supplemented with 2% (v/v) CO2 gas.
Determination of semi-continuous cultivation regime
The semi-continuous cultivation method was investigated under controlled laboratory scale conditions. The synthetic wastewater was used to provide a consistent nutrient composition without potentially toxic heavy metals or organic pollutants. Mono-species cultures were examined to determine the individual characteristics of selected microalgae for growth and protein accumulation.
A preliminary study was conducted to determine the strain-specific regime for the semi-continuous mode cultivation to sustain their exponential growth and to maximize the removal of nitrogen and phosphorus from the wastewater medium at the same time. The semi-continuous cultivation regime was determined individually for each strain by testing 1) the concentration of microalgae cells (initial cell density) to bring an exponential growth of the culture, and 2) the consumption of ammonium (NH4+) and phosphate (PO43–) in the culture medium.
For determination of suitable initial density of the culture, one culture was set up for each strain using the same cultivation condition as for experiment cultures (see “Microalgae and cultivation condition” for detail). The microalgae were grown using a repeated 24-hour-batch cultivation (semi-continuous cultivation) mode starting the cycles with different initial optical densities (OD). OD measurement was chosen as an indicator since it provided a quick estimation of cell density without complicated sample preparation and analysis. After each 24-h cultivation, a certain volume of the culture was taken out and the same volume of fresh medium was supplied according to the dilution rate needed for the cell density adjustment. The growth of the culture was measured by the increase of the OD values (the differences between the initial and the final OD). The consumption of NH4+ and PO43– was measured as the decrease of their concentration in the medium (see the Nutrient uptake section for details). An initial cell density was selected for the further experiment when the one resulted in a large OD increase, and in parallel, the NH4+ and PO43– concentration was not detectable at the end of the cycle (data not shown). A complete PO43– uptake was not observed within 24 h for K. flaccidum for any of the initial densities examined. So, the initial cell density for this strain was chosen only by the growth and NH4+ consumption. Accordingly, the initial OD for the semi-continuous cultivation regime was determined as 0.3 and 0.2 for L. segnis and K. flaccidum, respectively.
Experimental procedure
First, all experiment cultures were grown under batch cultivation mode until they consumed NH4+ in the wastewater medium completely. The semi-continuous cultivation regime was started on the day when NH4+ depletion was confirmed. For each strain, two different media were used for the semi-continuous cultivation, 1) complete medium, which contained all components (CM, n = 3) and deficient medium, in which NH4Cl and K2HPO4 were excluded (DM, n = 3). This latter medium treatment was used to study an effect of nitrogen (NH4+) and phosphorus (PO43–) depletion on the protein cell contents. The OD of each culture was measured to determine the volume of fresh medium, which needed to adjust to the predetermined initial OD. The determined volume was taken out from the culture, which included microalgae cells and medium (i.e. harvesting), and the same volume of fresh medium was added to the culture (described as “medium replacement” hereafter). Medium replacement was repeated every 24 h, and six cycles were investigated for each strain. The semi-continuous cultivation cycle was continued for the cultures that did not show increase in density. In this case, medium replacement was conducted based on the sampling volume needed for the analysis. The average initial OD was 0.31 ± 0.01 and 0.20 ± 0.02, which was equivalent to 387 ± 36 and 191 ± 27 x103 cells mL–1 for L. segnis and K. flaccidum, respectively (mean ± 1 SD, 6 cultivation cycles of 3 replicate cultures, Online Resource Table 1). Sampling was conducted before and after the medium replacement for the measurement of biomass production (OD and dry cell weight) and nutrient uptake (NH4+ and PO43–).
Biomass production and growth rate
Biomass production was measured using dry cell weight. For the determination of dry weight, the cells were separated from the medium using glass fiber filters (Whatman GF/A). The cells were washed with dH2O and were dried on the filter at 105 °C for 24 h. The growth was determined by increase in cell density, which was measured by optical density (OD) at 750 nm using a microplate reader (Spark, Techan, Switzerland). This wavelength was selected because it is outside of the absorption range of chlorophyll a, and thus, it would provide the least interference of microalgal pigments when measuring OD as an indication of cell concentration in suspended cultures (Griffiths et al. 2011). The specific growth rate (μ) was determined using the following equation, where N1 and N2 represent the OD750 values at times (days) t1 and t2 (Mayers et al. 2014):
Protein assay
The harvested culture was centrifuged at 3500 rpm for 10 min at 4 °C and the cells were pelletized by centrifuge at 15000 rpm for 2 min. The samples were freeze-dried and stored at –80°C. The total protein concentration was determined by Lowry colorimetric assay (Lowry et al. 1951) using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories) by the manufacturer’s protocol. Protein composition data were expressed as percentages of total dry cell weight (% dw).
Amino acid analysis
Amino acid profile was analyzed using the lyophilized freeze-dried samples collected at the end of the experiment (the 6th cycle). The analysis was conducted by a commercial lab using the method described in European Commission regulation (EC) No 152/2009 (EU 2009). Briefly, an oxidizing solution (750 mL L–1 formic acid and 4.73 g L–1 phenol) was added to the sample, which was then incubated for 17 h at 4 °C. Sodium sulfite was added and the samples were hydrolyzed with a hydrolysis solution (492 mL L–1 HCl and 1 g L–1 phenol) and incubated in the heating cabinet (110 °C) for 23 h. The pH of the samples was adjusted (2.0–2.4) and the samples were filtered (0.2 μm). Amino acids were separated by ion exchange chromatography and determined with a photometric detection at 570 nm (except proline at 440 nm) according to a reaction with ninhydrin (30+ Amino Acid Analyzer, Biochrom Ltd, England). For the total tryptophan determination, the samples were hydrolyzed in a saturated barium hydroxide solution at 110 °C for 20 h. The pH of the samples was adjusted to 6.2. The tryptophan concentration was determined by high performance liquid chromatography (Ultimate 3000 UHPLC system with autosampler, Thermo Scientific, and detector RF-535 fluorescence detector, Shimadzu). The reported amino acid values correspond to the concentrations in the dry weight of cells (g kg–1 dry cell weight), which were then converted to the percentage content in total dry cell weight (% dw). The results for aspartic acid concentration are presented as the sum of aspartic acid and asparagine, and these for glutamic acid are the sum of glutamic acid and glutamine. Tryptophan was not detectable in K. flaccidum because the extraction method was not sufficient to remove a presumable interfering substance.
Nutrient uptake
The culture medium was separated from the cells by centrifugation (3500 rpm for 10 min) and filtration (cellulose acetate membrane, 0.20 μm). The concentrations of NH4+ and PO43– in the samples were measured using an ion chromatography (940 Professional IC Vario, Metrohm, Switzerland) with the anion column Metrosep A Supp 5–150/4.0 and the cation column Metrosep C6–150/4.0 (Metrohm). The measurement was conducted using a cation eluent (1.7 mM 2,6– pyridinedicarboxylic acid and 1.7 mM HNO3) with a flow rate of 0.9 mL min–1, and an anion eluent (64 mM Na2CO3 and 20mM NaHCO3) with a flow rate of 0.7 mL min–1.
Statistical analysis
Experimental cultures were conducted in triplicate for each medium treatment (complete and deficient medium), and data for biomass production, protein content, and nutrient removal were collected for six semi-continuous cultivation cycles. Protein cell contents and biomass production were compared using one-way analysis of variance (ANOVA). Significant differences found by ANOVA were further analysed by multiple pair-wise comparison using Tukey’s honest significant differences (HSD) and compact letter displays. All data used for the one-way ANOVA met the assumption of normality and homogeneity of variance. They were checked with Shapiro-Wilk normality test and Levene’s test for homogeneity of variance. Statistical analyses were conducted using R software (version 4.2.2) (R Core Team 2022).
Results
Total protein contents
Lobochlamys segnis and K. flaccidum contained on average 62 ± 10 and 45 ± 4% dw (mean ± 1 SD) protein, respectively, under the semi-continuous cultivation regime using the complete medium. The highest protein cell content recorded during this study was 74 ± 7% dw for L. segnis, and 51 ± 1% dw for K. flaccidum (mean ± 1 SD). The protein cell content of L. segnis was similar in all cultures at the start (the cycle 0); however, it was significantly different (F13,28 = 134.1, p < 0.001) between the medium treatments after the commencement of the semi-continuous cultivation regime (Fig. 2a). This trend was observed also for K. flaccidum (F13,28 = 365.4, p < 0.001) (Fig. 2b). The lowest protein content in L. segnis and K. flaccidum was 17 ± 1 and 10 ± 1% dw, respectively, that were found among those grown with the deficient medium treatment.
Total proteins content (% of dry cell weight) in (a) Lobochlamys segnis and (b) Klebsormidium flaccidum under the semi-continuous cultivation regime using a complete medium (CM, black bar) and a medium deficient in nitrogen and phosphorus (DM, grey bar). The protein content was first measured before the start of the semi-continuous regime (cycle 0). Thereafter, it was measured in the cells that were harvested at the end of each cycle (cycle 1 to 6). Mean values ± 1 SD of 3 replicate cultures are shown (3 measurements from each). Different letters above the bars indicate significant differences (One-way ANOVA with Turkey’s HSD, p < 0.05)
A slight fluctuation of the protein proportion was observed for the complete medium treatment, although it was stabilized at the same level after the 4th cycle in both strains (Fig. 2a and b). In L. segnis, the protein cell content was reduced between the 1st and 3rd cycle, but it increased and stayed at a higher level (70%) later (Fig. 2a). For K. flaccidum, it was increased between the 2nd and 3rd cycle, but it was lowered and stabilized at 40% after the 4th cycle (Fig. 2b).
Biomass and protein productivity
The semi-continuous cultivation regime supported an active growth of both strains when using the complete medium. The specific growth rates (μ) of L. segnis were stable at 0.4 per day under the complete medium treatment, while a gradual reduction was shown under the deficient medium treatment (Fig. 3a). The K. flaccdum grown with the complete medium showed a fluctuation of the growth rate, although the rates were constantly higher than those that supplied with the deficient medium (Fig. 3b). Their growth rate reached the highest (0.7 ± 0.1 day–1) on the 6th cycle (Fig. 3b).
Specific growth rate (μ) day–1 based on the OD (at 750 nm) of (a) Lobochlamys segnis and (b) Klebsormidium flaccidum culture under the semi-continuous cultivation regime using a complete medium (CM, black indicators) and a medium deficient in nitrogen and phosphorus (DM, grey indicators). Mean values ± 1 SD (error bars) of 3 replicate cultures are shown (3 measurements from each)
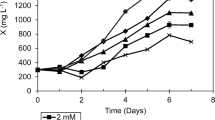
For both strains, the biomass production was relatively constant under the semi-continuous cultivation regime using the complete medium (Fig. 4a and b). The average biomass productivity was 572.5 ± 38.6 and 579.8 ± 49.0 mg L–1 day–1 for L. segnis and K. flaccidum, respectively (mean ± 1 SD). Conversely, reduction in the biomass production was evident with the deficient medium treatment (Fig. 4a and b). There was a significant difference in the cell dry weight between these medium treatments was shown already by the 2nd cultivation cycle for K. flaccidum (F13,28 = 35.09, p < 0.001) (Fig. 4b), while it was on the 4th cultivation cycle for L. segnis (F13.28 = 38.09, p < 0.001) (Fig. 4a).
Biomass production measured by dry cell weight (mg L–1) of (a) Lobochlamys segnis and (b) Klebsormidium flaccidum culture under the semi-continuous cultivation regime using a complete medium (CM, black bar) and a medium deficient in nitrogen and phosphorus (DM, grey bar). The biomass was measured at the start of the semi-continuous regime (cycle 0) and at the end of each cycle (cycle 1-6). Mean values ± 1 SD of 3 replicate cultures are shown (3 measurements from each). Different letters above the bars indicate significant differences (One-way ANOVA with Turkey’s HSD, p < 0.05)
The overall protein productivity achieved by the semi-continuous cultivation strategy was 351.0 ± 34.5 mg L–1 day–1 for L. segnis and 257.7 ± 33.7 mg L–1 day–1 (mean ± 1 SD) for K. flaccidum.
Medium replacement rate, nutrient removal, and pH
For the cultures with the complete medium, the medium replacement rate was 21 ± 3 and 24 ± 4% day–1 (mean ± 1 SD) for L. segnis and K. flaccidum, respectively (Table 1). The deficient medium did not support culture growth after the 4th cycle for any of the strains (Online Resource Fig. 2a and b) and the regime based on the initial cell density adjustment could not be continued. Instead, a fixed volume of the medium was harvested and replaced for the deficient medium cultures. The harvest/medium replacement volume after 5th cycle was 50 mL (8%) for L. segnis and it was 60 mL (18%) at the 5th cycle and 100 mL (25%) at the 6th cycle for K. flaccidum (Table 1). A larger harvesting volume was needed for K. flaccidum culture to obtain enough biomass for the analysis.
Under the initial batch mode cultivation, complete uptake of NH4+ was observed on day 3 and day 5 for L. segnis and K. flaccidum, respectively (Fig. 5a and b). When the semi-continuous mode had started, NH4+ and PO43- that supplied by fresh medium were completely consumed within a single cycle (24 h) in the L. segnis culture (Fig. 5a and c). The average removal rate of NH4+ was 9.2 ± 0.9 mg L–1 day–1 and of PO43- was 5.2 ± 0.9 mg L–1 day–1 (mean ± 1 SD). Klebsormidium flaccidum also showed a complete uptake of NH4+ in each cycle under the semi-continuous mode cultivation, resulting a removal rate of 13.8 ± 3.2 mg L–1 day–1, which was higher than that for L. segnis (Fig. 5b). However, the PO43- uptake was limited to an average 24 ± 14% of the daily supplement (5.1 ± 1.3 mg L–1 day–1), therefore, PO43- was partially accumulated in the culture medium over time (Fig. 5d).
Nutrient removal under the semi-continuous cultivation regime using a complete medium (CM, black full line) and a medium deficient in nitrogen and phosphorus (DM, grey dotted line). Changes of the NH4+ concentrations in (a) Lobochlamys segnis and (b) Klebsormidium flaccidum culture medium. Changes of the PO43- concentrations in (c) L. segnis and (d) K. flaccidum culture medium. The nutrient concentrations were measured both at the start and the end of each cultivation cycle (C1-C6). The commencement of the semi-continuous regime for L. segnis and K. flaccdium was on day 3 and day 5, respectively, when a complete NH4+ consumption was confirmed. Mean values ± 1 SD (error bars) of 3 replicate cultures are shown (3 measurements from each)
The pH measured at the end of each semi-continuous cultivation cycle slightly fluctuated (Fig. 6a and b), but the variation range was limited between 7 and 8.5. Constant medium replacement in along with CO2 gas supplementation was sufficient to control the medium pH in an acceptable range.
The medium pH of (a) Lobochlamys segnis and (b) Klebsormidium flaccidum culture under the semi-continuous regime using a complete medium (CM, black indicators) and a medium deficient in nitrogen and phosphorus (DM, grey indicators). Mean values ± 1 SD (error bars) of 3 replicate cultures are shown (3 measurements from each)
Amino acids
The amino acid compositions and proportions of single amino acids were relatively similar in both strains (Online Resource Fig. 3). The most abundant amino acid was glutamic acid, where L. segnis contained 4.2 ± 0.0% dw and K. flaccidum contained 4.7 ± 0.2% dw (mean ± 1 SD) (Table 2). All essential amino acids were found, except for the tryptophan in K. flaccidum. Among the essential amino acids, leucine, arginine, and lysine occurred as the major components. The compositions of these amino acids were 3.5 ± 0.3, 3.4 ± 0.4, 2.8 ± 0.1% dw in L. segnis and 2.7 ± 0.0, 2.5 ± 0.1, 2.2 ± 0.0% dw in K. flaccidum, respectively (Table 2).
Discussion
Effect of semi-continuous cultivation for protein production
The semi-continuous cultivation regime we designed maintained active growth with associated protein production of L. segnis F12 and K. flaccidum NIVA-CHL80. The regime was designed individually for each strain by their growth rate (cell density increase), and nitrogen requirements (ammonium uptake). This regime assured that each cycle started with a suitable number of cells capable for exponential multiplication with constant access to sufficient nutrients. Such cell density regulation also provided optimal light penetration for the culture. Generally, the density of microalgae culture largely affects light availability, both in terms of quality and quantity, which is one of the major limitation factors for productivity (Borowitzka 2016). Importantly, our result demonstrated the effect of nitrogen and phosphorus availability on protein proportions in cells. Both strains showed a considerable reduction in biomass (Fig. 4) and protein contents (Fig. 2) when the semi-continuous cultivation regime was conducted using the deficient medium (i.e., lacking NH4+ and PO43-). Comparing the deficient to the complete medium, even during the first cycle, cellular protein contents were significantly reduced (Fig. 2), despite similar or higher (in the case of L. segnis) growth rates (Fig. 3). Microalgae respond to nutrient limitations by decreasing cell division and photosynthesis (Procházková et al. 2014). They also activate a “nutrient scavenging system”, where nitrogen is recycled by degrading intracellular proteins (Procházková et al. 2014). We hypothesized that in our system, during the first cycle of the deficient medium treatments, nitrogen limitation triggered such “nutrient scavenging system” to maintain active cell multiplication, and this may be responsible for the significant decrease in cellular protein content. Subsequently, culture growth slowed after the second cycle presumably because the cells were no longer viable enough to keep up a high multiplication rate.
This study also demonstrated that constant nitrogen supply can stabilize protein levels in the studied strains. In our previous study the total proteins contents of these strains gradually decreased over a batch cultivation period (Umetani et al. 2023). The importance of nitrogen availability for the maintenance of high cellular protein content was also suggested by a previous study of a freshwater macroalga (Cole et al. 2015). These authors provided a weekly supply of nitrogen for the cultivation of Oedogonium sp., resulting in higher proportion of protein compared to experimental treatments that received less frequent nitrogen supplies. Interestingly, their study also found that the periodic nitrogen resupply increased protein levels, regardless of the durations of nitrogen starvation. Coupled with our results, this strongly suggests that a semi-continuous cultivation strategy should be designed according to the nitrogen requirement of the microalgae species of interest. Such a custom-made regime would thus maintain an optimum protein content of the microalgae species of interest.
Moreover, our results point out an advantage of the semi-continuous cultivation approach over batch cultivation regarding biomass productivity. K. flaccidum took a longer time to increase density during the first batch cultivation period (day 0-5, Online Resource Fig. SI1b) when compared to L. segnis (day 0-3, Online Resource Fig. SI1a). Despite the late start, K. flaccidum multiplied rapidly (Fig. 3b) and produced a biomass similar to L. segnis (Fig. 4) under the semi-continuous cultivation. The results implies that the semi-continuous cultivation regime induces exponential growth of K. flaccidum without the long lag phase that is observed in the batch cultivation modus. Both L. segnis and K. flaccidum showed a slight fluctuation in the protein cell contents between 1st and 3rd cycle, that became stable after the 4th cycle (Fig. 2). An average of 21% and 24% of L. segnis and K. flaccidum culture was harvested in each cycle of the cultivation regime (Table 1), therefore in theory, around roughly the 4th– 5th cycle was when all culture in the batch cultivation mode was replaced. This may indicate an acclimation process is needed for optimal microalgae performance for the semi-continuous cultivation mode. In our system, acclimated vital cells displayed and continued the high rate of multiplication under desirable conditions of light and nutrient. This is important as enhancement of biomass productivity is a key criteria to optimize overall protein productivity (Geada et al. 2021). We therefore suggest that there are strong advantages to the semi-continuous cultivation approach over batch cultivation and incorporating appropriate acclimation periods is an important consideration. This framework is applicable for protein production of strains with a range of growth characteristics.
Nutrient removal efficiency
This study also aimed to test if the semi-continuous regime was effective for nutrient removal from the wastewater. L. segnis performed complete removal of NH4+ and PO43– that was supplied at the start of each cycle. Also, K. flaccidum showed the same efficiency for NH4+, although total PO43– removal was not achieved. Thus, L. segnis was effective for both nitrogen and phosphorus nutrient removal, while K. flaccidum only succeeded in NH4+ removal. Wastewater nutrient composition varies according to the types of waste streams, and the nutrient removal efficiency differs among chlorophyte species (Aggarwal and Remya 2022). Because of this, when developing a semi-continuous cultivation approach and regime for wastewater treatment, adjustment will be required according to the range of concentration of nitrogen and phosphorus in a specific wastewater, as well as the nutrient uptake characteristics of target microalgae species. Thus, microalgae taxon-specific treatment efficiency in specific wastewater nutrient concentration scenarios should be evaluated in further studies along with the effects of possible growth inhibitors likely to be present in waste streams.
Our semi-continuous cultivation regime was designed for a maximize usage of nutrients in the simulated wastewater, resulting in efficient nutrient removal. This aim is unlike general microalgae cultivation practice, where maximum biomass or target cell compound generation would be prioritized rather than full utilization of nutrients. With the main purpose of complete usage of nitrogen, we argue that protein would be better suited as the target among other potential commodities. Our results showed that constant nitrogen supply optimizes protein production. Such a nitrogen requirement is congruent with the demand of continuous removal of excess nutrients, especially of ammonium nitrogen in wastewater treatment practice. When applying the semi-continuous cultivation regime, untreated wastewater containing nitrogen source could be supplied regularly for microalgae, which would consequently facilitate a production of high share of protein content. Other microalgae cell compounds of interests, such as carbohydrates and lipids, are storage energy products that often employ nutrient deficient treatments for their production enhancement (Liyanaarachchi et al. 2021). For these compounds the need of nutrient deficiency would require a period of reduction in the inflow of untreated wastewater, relatively minimizing the wastewater treatment capacity compared to protein production.
Protein and amino acid profiles in the studied strains
The studied strains showed their potential as model protein production species. With the semi-continuous cultivation regime, the average total protein content of L. segnis and K. flaccidum, was 62 and 45% dw, respectively. Thus, from our strains, L. segnis F12 was the best candidate for further study. The protein contents of the studied strains were comparative or higher in comparison to those reported for commercially produced microalgae species, such as Arthrospira spp. (42-75%), Chlorella spp. (42-68) and Euglena sp. (50-70%) (Ritala et al. 2017), also to those recently studied species for protein production, for example Chlorella vulgaris (67%) (Seelam et al. 2022) and Galdieria sulphuraria (44%) (Montenegro-Herrera et al. 2022). Lobochlamys segnis and K. flaccidum general protein levels are also comparable to those of fungi (30-50%) and bacteria (50-80%) (Ritala et al. 2017).
The protein quality in terms of amino acid profiles was similar between the studied strains. The predominance of glutamic acid was similar to the previously reported characteristic of freshwater and marine microalgae proteins that have been studied for animal and aquaculture feeds (Phaeodacylum tricornutum, Nannochloropsis granulata, Botryococcus braunii, Porphyridium aerugineum, Tetraselmis chuii) (Tibbetts et al. 2015). The essential amino acid compositions in the studied strains, especially lysine as a major component, could be advantageous for feed applications. Among the essential amino acids, lysine and methionine are typically limiting in animal feeds and these are usually supplied in the form of synthetic crystalline amino acid (Tibbetts et al. 2015; Fawcett et al. 2022). Tryptophan has been found primarily as a minor component in microalgae, but difficulty in its detection has also been reported previously for other microalgae species, including Galdieria suphuraria (Montenegro-Herrera et al. 2022). Further development of a reliable tryptophan analysis method is crucial to achieve in-depth evaluation of microalgae amino acid profile for food and feed applications.
Future perspectives and challenges
The studied strains contained a high share of protein with an appealing amino acid profile and should be further studied for relevant application in e.g. animal feed supplements and fish feed ingredients. Microalgae protein production could potentially provide other benefits in addition to protein, such as polyunsaturated fatty acids, antioxidants, vitamins, and minerals (Tibbetts et al. 2019; Jones et al. 2020; Fawcett et al. 2022). For their protein to be considered as feed ingredients, further evaluations of digestibility and bioavailability are required (Tibbetts et al. 2019) as will be evaluation of possible adverse effect of toxins and pathogens. Other possible usage of microalgae protein and amino acids may be in biofertilizer and biostimulant applications (Braun and Colla 2023).
Similarly, microalgae protein production using waste streams for human nutrition will meet significant regulatory challenges (Fernández et al. 2021), and microalgae cultivation conditions for feed application are also regulated by “good manufacturing and agricultural practices” for product safety (Janssen et al. 2022). This study used the simulated nutrition components of municipal wastewater; however, a broader range of real waste streams will require investigation to assess overall relevance in these applications. For example, food-processing wastewaters containing low levels of toxic heavy metals and other contaminants may be a more reliable source medium for protein production for animal feed (Vethathirri et al. 2021). This study also has limited insights into economic aspects of the proposed cultivation method, and thus it should be a focus of future research to scale-up for adaptation of the semi-continuous cultivation, as well as the optimization of nutrients from real wastewater. Evaluations should be conducted in consideration of downstream operational costs for harvesting and processing, such as biomass stabilization and protein extraction. There still is a long path towards establishing microalgae protein production combined with wastewater treatment. But our results indicate the positive potential of, and a path forward for, further research towards the dual benefits of combined sustainable microalgae protein production and wastewater treatment.
Conclusion
The semi-continuous cultivation system we designed supported a stable protein-rich biomass production in L. segnis and K. flaccidum. The designed regime based on fixed initial cell density of the cultivation cycle assured exponential culture growth in each cycle with a consequent high cellular protein proportion. The cultivation strategy also effectively removed both nitrogen and phosphorus, or just nitrogen, from the wastewater medium. Proteins obtained from the studied strains showed an amino acid profile favorable for feed application. This study established a basic cultivation method for the enhancement of protein cell contents and for the efficient use of nutrients in the municipal wastewater. Our results support further development of combined microalgae-based protein production and wastewater treatment systems.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acién FG, Fernández JM, Molina-Grima E (2014) Economics of microalgae biomass production. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 313–325
Aggarwal M, Remya N (2022) The state-of-the-art production of biofuel from microalgae with simultaneous wastewater treatment: influence of process variables on biofuel yield and production cost. BioEnerg Res 15:62–76
Akmukhanova NR, Sadvakasova AK, Torekhanova MM, Bauenova MO, Zayadan BK, Shalgimbayeva SM, Bolatkhan K, Alwasel S, Leong YK, Chang J-S, Allakhverdiev SI (2022) Feasibility of waste-free use of microalgae in aquaculture. J Appl Phycol 34:2297–2313
Avila-Leon I, Chuei Matsudo M, Sato S, de Carvalho JCM (2012) Arthrospira platensis biomass with high protein content cultivated in continuous process using urea as nitrogen source. J Appl Microbiol 112:1086–1094
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Bischoff HW, Bold HC (1963) Some soil algae from enchanted rock and related algal species. Phycological Studies, University of Texas IV:1–95
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Cham, pp 601–652
Braun JCA, Colla LM (2023) Use of microalgae for the development of biofertilizers and biostimulants. BioEnerg Res 16:289–310
Cole AJ, Angell AR, de Nys R, Paul NA (2015) Cyclical changes in biomass productivity and amino acid content of freshwater macroalgae following nitrogen manipulation. Algal Res 12:477–486
EU (2009) Commission Regulation (EC) No 152/2009. Laying down the methods of sampling and analysis for the official control of feed. Annex III, F Determination of amino acids. pp 19–24
Fawcett CA, Senhorinho GNA, Laamanen CA, Scott JA (2022) Microalgae as an alternative to oil crops for edible oils and animal feed. Algal Res 64:102663
Fernández FGA, Reis A, Wijffels RH, Barbosa M, Verdelho V, Llamas B (2021) The role of microalgae in the bioeconomy. New Biotechnol 61:99–107
Fuentes-Grünewald C, Ignacio Gayo-Peláez J, Ndovela V, Wood E, Vijay Kapoore R, Anne Llewellyn C (2021) Towards a circular economy: a novel microalgal two-step growth approach to treat excess nutrients from digestate and to produce biomass for animal feed. Bioresour Technol 320:124349
Geada P, Moreira C, Silva M, Nunes R, Madureira L, Rocha CMR, Pereira RN, Vicente AA, Teixeira JA (2021) Algal proteins: production strategies and nutritional and functional properties. Bioresour Technol 332:125125
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Meth 85:119–123
Janssen M, Wijffels RH, Barbosa MJ (2022) Microalgae based production of single-cell protein. Curr Opin Biotech 75:102705
Jones SW, Karpol A, Friedman S, Maru BT, Tracy BP (2020) Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr Opin Biotech 61:189–197
Kotai J (1972) Instructions for preparation of modified nutrient solution Z8 for algae, Publication B-11/69. Norwegian Institute for Water Research, Oslo, Norway
Liyanaarachchi VC, Premaratne M, Ariyadasa TU, Nimarshana PHV, Malik A (2021) Two-stage cultivation of microalgae for production of high-value compounds and biofuels: a review. Algal Res 57:102353
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mayers JJ, Flynn KJ, Shields RJ (2014) Influence of the N:P supply ratio on biomass productivity and time-resolved changes in elemental and bulk biochemical composition of Nannochloropsis sp. Bioresour Technol 169:588–595
Montenegro-Herrera CA, Vera-López Portillo F, Hernández-Chávez GT, Martinez A (2022) Single-cell protein production potential with the extremophilic red microalgae Galdieria sulphuraria: growth and biochemical characterization. J Appl Phycol 34:1341–1352
Muys M, Sui Y, Schwaiger B, Lesueur C, Vandenheuvel D, Vermeir P, Vlaeminck SE (2019) High variability in nutritional value and safety of commercially available chlorella and Spirulina biomass indicates the need for smart production strategies. Bioresour Technol 275:247–257
Nagarajan D, Lee D-J, Chen C-Y, Chang J-S (2020) Resource recovery from wastewaters using microalgae-based approaches: a circular bioeconomy perspective. Bioresour Technol 302:122817
Oss RN, Gonçalves RF, Cassini ST, Junior MÂS, Cipriano DF, de Freitas JCC (2022) Single step production of activated carbon from microalgae cultivated with urban wastewater. Algal Res 64:102669
Procházková G, Brányiková I, Zachleder V, Brányik T (2014) Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J Appl Phycol 26:1359–1377
Pröschold T, Marin B, Schlösser UG, Melkonian M (2001) Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist 152:265–300
R Core Team (2022) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Ritala A, Häkkinen ST, Toivari M, Wiebe MG (2017) Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front Microbiol 8:2009
Schwenzfeier A, Wierenga PA, Gruppen H (2011) Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour Technol 102:9121–9127
Seelam JS, Fernandes de Souza M, Chaerle P, Willems B, Michels E, Vyverman W, Meers E (2022) Maximizing nutrient recycling from digestate for production of protein-rich microalgae for animal feed application. Chemosphere 290:133180
Seyfabadi J, Ramezanpour Z, Amini Khoeyi Z (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726
Show PL (2022) Global market and economic analysis of microalgae technology: status and perspectives. Bioresour Technol 357:127329
Tibbetts SM, Milley JE, Lall SP (2015) Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 27:1109–1119
Tibbetts SM, Patelakis SJJ, Whitney-Lalonde CG, Garrison LL, Wall CL, MacQuarrie SP (2019) Nutrient composition and protein quality of microalgae meals produced from the marine prymnesiophyte Pavlova sp. 459 mass-cultivated in enclosed photobioreactors for potential use in salmonid aquafeeds. J Appl Phycol 32:299–318
Tiron O, Bumbac C, Manea E, Stefanescu M, Nita Lazar M (2017) Overcoming microalgae harvesting barrier by activated algae granules. Sci Rep 7:4646
Tiron O, Bumbac C, Patroescu IV, Badescu VR, Postolache C (2015) Granular activated algae for wastewater treatment. Water Sci Technol 71:832–839
Umetani I, Sposób M, Tiron O (2023) Indigenous green microalgae for wastewater treatment: nutrient removal and resource recovery for biofuels and bioproducts. BioEnerg Res. https://doi.org/10.1007/s12155-023-10611-9
Vethathirri RS, Santillan E, Wuertz S (2021) Microbial community-based protein production from wastewater for animal feed applications. Bioresour Technol 341:125723
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotech 5:34
Wu GY, Li P (2022) The "ideal protein" concept is not ideal in animal nutrition. Exp Biol Med 247:1191–1201
Acknowledgements
The authors would like to thank Dr. Glenn Dunshea for comments that improved the manuscript as well as proof reading and language editing.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research The research leading to the results has received funding from the NO Grants 2014-2021, under Project contract no. 27/2020.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. I. Umetani performed material preparation and data collection. All authors involved in the analyses and interpretation of the results. The draft of the manuscript was written by I. Umetani, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 511 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umetani, I., Sposób, M. & Tiron, O. Semi-continuous cultivation for enhanced protein production using indigenous green microalgae and synthetic municipal wastewater. J Appl Phycol 36, 1105–1116 (2024). https://doi.org/10.1007/s10811-023-03179-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03179-6