Abstract
Intertidal seaweed beds form three-dimensional structures providing habitat for a variety of species. As such, ecosystem-based management of seaweed harvesting must take into consideration the impact of the harvest not only on the biomass but also on the morphology of the seaweed. We compare the morphology and vertical distribution of biomass and shoots in Ascophyllum nodosum from three sites with a 20 + year history of commercial harvesting with three corresponding control sites in southern New Brunswick, Canada. We found no significant impact of harvest history on the vertical distribution of shoots or biomass within individual clumps. At two of the three harvested sites, large clumps had a wider circumference than those at the control sites, suggesting that long-term harvesting increases the growth of shoots throughout the clumps; presumably caused by an increase in light penetration through the harvested canopy. We also compare biomass of littorinids, the most abundant invertebrates found in A. nodosum beds at low tide and found no significant difference between control and harvested sites. We conclude that the harvest of A. nodosum according to the current regulations in New Brunswick, does not have long-term impact on the morphology of the algae or on the abundance of its main inhabitant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global demand for macroalgae is in constant increase worldwide. While most of the supply comes from the aquaculture sector, mainly from Asia, wild harvest continues to be an important source of seaweed in Europe, North America and South America (FAO 2021). Wild harvest plays an important role in providing livelihood in many coastal communities (Rebours et al. 2014). With wild stock being by nature limited and, in some cases, already fully exploited or overexploited (Vásquez 2008), it becomes increasingly important to ensure that adequate management is in place to maintain the sustainability of the harvest as pressure increases. Many large seaweeds are foundational species that play important ecological roles in shaping communities (Steneck and Johnson 2014) and provide valuable ecosystem services (Eger et al. 2023). Harvesting activities can temporarily alter the structure of seaweed beds and consequently impact their functions in the ecosystem (Steen et al. 2016; Norderhaug et al. 2020). As such, kelp forests and intertidal and subtidal seaweed beds must be managed through an ecosystem-based management (Ugarte and Sharp 2001; Lotze et al. 2019).
While vegetated marine areas tend to harbour a greater diversity of species than denuded areas, specific assemblages are in part dependent on macroalgal morphology (Torres et al. 2015; Gan et al. 2019; Lemay et al. 2021). The role of seaweed architectural complexity as a predictor of invertebrate communities is unclear as even congeneric algae with similar forms can harbour very different diversity of invertebrate assemblage (Wikström and Kautsky 2004; Bates 2009). Furthermore, the role of algal morphological complexity on invertebrate assemblages can vary across scale, from the branching pattern of individual thalli (Chemello and Milazzo 2002) to the distribution of individuals in space, the heterogeneity of thalli arrangements, and the fragmentation of algal beds (Roberts and Poore 2006). To that effect, while algal canopy increases alpha diversity it can also reduce spatial variation of temperature and desiccation during low tide, leading to a decrease in understory beta diversity of sessile organisms (Scrosati et al. 2021; Catalán et al. 2023). It becomes difficult to predict how harvesting of various seaweeds under a range of management strategies will impact communities, especially without a better understanding of the magnitude of morphological changes at the thallus and bed levels caused by the harvest.
In North America and parts of Europe, the brown seaweed, Ascophyllum nodosum (Fucales, Phaeophyceae, hereafter Ascophyllum), is the principal seaweed species harvested by volume. It is widely distributed on rocky shores in the North Atlantic (Pereira et al. 2020) and is commercially harvested in Canada, USA, Norway, Iceland, Ireland, Scotland, and France. As an easily accessible intertidal species, it has a long history of being used as a soil amendment (Guiry and Morrison 2013) and as an animal food supplement (Morais et al. 2020). In recent years, its recognition as a powerful plant biostimulant has led to an increase in demand (Shukla et al. 2019).
Contrary to other commonly harvested species (e.g., Laminaria hyperborea, L. digitata), which do not regrow following harvest because the meristem is removed, Ascophyllum will regrow if cut above the holdfast as it is a modular alga where several fronds can grow from a common holdfast (Lazo and Chapman 1998). A frond originates from a single shoot at the holdfast and is comprised of primary and lateral shoots as well as reproductive receptacles (Åberg 1989). A shoot is defined as any branching element of the frond. Individuals in Ascophyllum represent the assemblage of fronds and shoots arising from a common holdfast (Baardseth 1955). Holdfasts can fragment and holdfasts from different individuals can coalesce over time, making it nearly impossible to differentiate individuals in Ascophyllum (Åberg 1989). We define a clump as the assemblage of shoots and fronds originating from one or several touching individual holdfasts. Therefore, a clump may be formed from a single or several individuals.
One of the concerns regarding the harvest of Ascophyllum is that while the biomass and canopy height of the beds may be preserved (Lauzon-Guay et al. 2021), repeated cutting may lead to increase lateralisation (Boaden and Dring 1980) and individuals may become “bushier” (Seeley and Schlesinger 2012). Morphological characteristics of Ascophyllum at the bed level from different sites or regions have been linked to overall community compositions (Kay et al. 2016a). The extent to which local communities may be affected by changes in Ascophyllum morphology or the level of morphological changes required for communities to be impacted is yet to be resolved. In the present study we evaluate the impact of long-term harvesting on the morphology and vertical distribution of shoots and biomass in Ascophyllum in New Brunswick, Canada. We compare morphological characteristics of Ascophyllum as well as associated snail biomass at sites that have been commercially harvested for over two decades with never harvested sites that have been closed to the harvest ever since the advent of commercial harvesting in the province. This provides a unique opportunity to assess the long-term impact of harvesting activities on Ascophyllum morphology.
Materials and methods
Study location
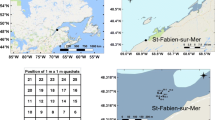
All sites are located in the Bay of Fundy in southern New Brunswick, Canada. Site selection was based on the availability of Ascophyllum beds of commercial interest but closed to commercial harvesting in proximity to beds that have been actively harvested for several years (> 20 years). Three closed areas were selected, the Barnes-Simpson-Mowat Islands (BSM) Long-Term Reference Area (LTRA), the Green Point (GP) Study Site and the Maces Bay (MB) LTRA (Fig. 1). Both the Study Site and the LTRAs have been closed to commercial harvesting of Ascophyllum since the onset of the harvest in 1995 by the New Brunswick Department of Agriculture, Aquaculture and Fisheries (Ugarte and Sharp 2001). One commercially harvested bed within 1.2 km each of the three closed areas was selected. These beds have been harvested on a near continual basis for over 20 years. Only sections of the bed are harvested on any particular year, for this study, transects were laid in areas that did not contain any signs of a recent harvest. The purpose of this study was to see the long-term impact of a repeated harvest rather than the initial impact after a harvest.
Field sampling
At each site, a 30-m transect was deployed parallel to the shore in the mid-intertidal within an Ascophyllum bed, the area where the harvest is concentrated. Twenty 25 × 25 cm quadrats were randomly positioned along the transect. Each Ascophyllum clump, defined as all fronds and shoots originating from a single holdfast, with more than half their holdfast within the quadrat were removed from the substrate using a putty knife just below the holdfast. Prior to removing a clump, all shoots were tied together using a plastic cable-tie to prevent pieces of individual holdfast or coalesced holdfast to break apart during transport. All clumps from a quadrat were put in a marked plastic bag and brought back to the lab for further analysis. All snails present on the substrate within the quadrat or found on the Ascophyllum back in the lab were weighed. Two species of littorinids were found: Littorina littorea and Littorina obtusata. Because of the very low abundance of the latter, data for both species were pooled. Sampling was carried out on 19–20 October 2019, as the outside temperature was cool (< 10 °C) the samples were not put on ice for transport but were placed in a shaded location. Samples were refrigerated upon returning to the lab and were processed within 24 h. A small sample of clumps was collected in 1996 and 2001 prior to the large scale expansion of commercial harvesting of Ascophyllum in New Brunswick (Supplementary Material).
Sample processing
Each clump was measured for weight, height, and maximum circumference. Each clump was laid flat on a table, and the holdfast (1 cm section) was cut-off. The rest of the clump was divided into 10-cm sections starting just above the holdfast. Each section was weighed individually, and the number of shoots were counted in every second section, starting from the first one because of time constraints (i.e. shoots in sections 1, 3, 5, 7, etc. were counted).
Analysis
Clumps were divided into six size classes based on clump height (< 40 cm, 40–60 cm, 60–90 cm, 90–110 cm, 110–130 cm, and > 130 cm, following Ugarte et al. 2006). The proportional distribution of biomass within each section was calculated by dividing the weight of each section by the total weight of the clump. Similarly, the proportional distribution of shoots was calculated by dividing the number of shoots within a section by the sum of all shoots in all counted sections. Averages were calculated for each size class at each site. A value of zero was included for any clump that had no shoot in a specific section. To assess whether the harvest had an impact on the distribution of the biomass and on branching patterns, we calculated three indices. The first index was calculated as the height corresponding to the mid-point of the biomass distribution for each clump. This is the height that divides the clump into two halves of equal mass. The second index was calculated as the ratio of the biomass in the section located approximately 1/3 up the clump to the biomass in the section located approximately 2/3 up the clump. This index was used to evaluate whether the harvest caused a shift in the distribution of the biomass within the frond. We used these proportional heights instead of fixed height to be able to compare all size classes. Potentially, the harvest could increase the biomass in the lower section vs the upper section. The third index was similar to the second but used the number of shoots in the sections located 1/3 and 2/3 up the clumps to calculate a ratio of number of shoots. This index was used to evaluate whether the harvest had an impact on the branching pattern along a clump.
Statistical analysis
Vertical distribution of biomass and shoots between harvested and control sites were compared for each region for each size class using a two-sample Kolmogorov–Smirnov Goodness-of-Fit test. Morphological indices were analysed for each area separately using two-way ANOVAs with size class and treatment (harvested vs control) as fixed effects. If significant effects were detected, multiple comparisons were used to evaluate in which size classes the difference between harvested and control sites was significant. Average clump height was analysed using a two-way ANOVA with area and treatment as fixed factors. Clump circumference was compared between treatments using ANCOVAs with clump height as a covariate. In the case of non-homogeneity of slopes, the Johnson-Neyman technique was used to delineate the zone of non-significance (White 2003). Snail biomass was analysed using a two-way ANOVA with area and treatment (harvest vs control) as fixed effects. Data were log-transformed to meet the homoscedasticity and linearity assumptions. Data are presented as mean ± standard error. All statistical analyses were done in R v4.2.0 (R Core Team 2020).
Results
Average clump weight generally increased with size class and was similar between harvested and control sites (Fig. S1). An effect of harvesting was only statistically significant at Maces Bay where clumps at the historically harvested site were heavier than those in the control site (F1,75 = 6.874, p = 0.011).
Average clump height varied between 72.9 and 92.2 cm across all sites and there was no significant interaction between areas and treatment (F2,383 = 1.953, p = 0.143) and no significant difference between areas (F2,383 = 2.373, p = 0.095). There was a significant effect of treatment (F1,383 = 6.303, p = 0.013) with clumps in harvested sites (82.9 ± 5.6 cm) being on average taller than clumps in control sites (76.9 ± 2.4 cm).
Clump circumference increased with clump height in all areas (Fig. 2). At BSM, the slopes of the regressions differed between harvested and control sites (F2,140 = 7.976, p = 0.005). Circumference was lower at the harvested site for clumps shorter than 41.0 cm, while circumference was greater at the harvested site for clumps longer than 84.3 cm. There was no difference in circumference between harvested and control sites for clump of intermediate height (41.0 to 84.3 cm). At GP, there was no effect of treatment on the intercept of the regressions (F1,115 = 2.675, p = 0.105), while at MB, clumps at the harvested site on average had a greater circumference than those at the control site (F1,98 = 7.465, p = 0.007). Back-transformed adjusted means for the MB site are 8.08 ± 1.08 cm and 10.91 ± 1.08 cm for clumps from the control and the harvested site respectively.
The vertical biomass distribution differed between size classes. For the two smallest size classes (< 40 and 40—60 cm), the first Sect. (0–10 cm) contained the most biomass and the proportion of biomass decreased monotonically towards the tip of the clumps (Fig. 3). For intermediate size-classes, the biomass was more uniformly distributed with more biomass located in the center of the clump rather than near the holdfast. In larger size-classes the first Sect. (0–10 cm) was not the largest contributor to the biomass and biomass was more uniformly distributed along the height of the clump (Fig. 3). There was no significant difference between the shape of the distributions between harvested and control sites for any of the size classes in any areas (Table S1). Similar morphological patterns were observed in clumps collected prior to the onset of large-scale commercial harvesting in New Brunswick (Fig. S3). The analysis could not be performed for the > 130 cm size class in MB because only two clumps of that size were found at the control site and none at the harvested site (Table S2). The absence of significant differences in the vertical distribution of biomass between harvested and control sites is supported by the lack of significant differences between harvested and control site for the mid-biomass height (Fig. 4). The mid-biomass height increases monotonically with size-classes and was significantly higher at the harvested than at the control site at BSM (F1,114 = 5.469, p = 0.021). There was no significant interaction or effect of treatment at the other two sites. There was a significant effect of size class but no effect of treatment of the ratio of weight at 1/3 vs 2/3 of the clump height at all sites (Fig. S2). At BSM, the effect of treatment was marginally non-significant (F1,114 = 3.744, p = 0.055), with a higher ratio at the control than the harvested site. Generally, the ratio decreased monotonically with size classes. Small clumps can have more than 15 times more biomass in the lower than the upper section, while this ratio approaches unity for larger clumps, indicating that the biomass is uniformly distributed along the clump.
Average (+ SE) proportion of Ascophyllum nodosum clump biomass divided in 10-cm sections by size classes for harvested (light gray) and control (black) sites in three regions of southern New Brunswick (A. Green Point, B. BSM Islands, C. Maces Bay. Section 1 represent the section nearest the holdfast
The vertical distribution of shoots along clumps followed a similar pattern as the biomass. More shoots were found near the holdfast for the smallest size classes and decreased monotonically toward the tip of the clumps (Fig. 5). While the first section (0–10 cm) still held the most shoots in larger size classes, a second mode was found near the center of the clump, particularly in MB (Fig. 5). There was no significant difference in the distribution pattern between control and harvested sites (Table S1). A greater proportion of shoots were found in the bottom 1/3 section that the 2/3 section (Fig. 6). The shoot ratio was greater for smaller size classes than for larger size classes and no significant difference could be detected between control and harvested sites although it was marginally non-significant at BSM (F1,114 = 2.830, p = 0.095) with a higher ratio at the control than at the harvested site. While this is not statistically significant, it suggests that there may proportionally be more shoots near the base of the clumps than at the top at the control site compared to the harvested site. For smaller size classes there could more than 10 times more shoots in the bottom section than the upper section, while for larger clumps, the ratio approached unity, indicating that the shoots are more uniformly distributed along the height of the clumps in large clumps.
Average (+ SE) proportion of Ascophyllum nodosum clump shoots divided in 10-cm sections by size classes for harvested (light gray) and control (black) sites in three regions of southern New Brunswick (A. Green Point, B. BSM Islands, C. Maces Bay). Section 1 represent the section nearest the holdfast. Shoots were counted in every second 10-cm section
Both Littorina littorea and L. obtusata were present at all sites, with the former being much more abundant. There was a significant difference in total snail biomass between areas (F2,51 = 5.00, p = 0.010) with BSM islands (8.85 ± 3.43 g (0.0625 m)−2) having lower snail biomass than Maces Bay (19.03 ± 2.00 g (0.0625 m)−2; t51 = -3.027, p = 0.011). Littorinids biomass was intermediate at Green Point (14.47 ± 2.57 g (0.0625 m)−2). There was no significant interaction between areas and treatment (F2,51 = 0.030, p = 0.970) or significant effect of treatment (F1,51 = 0.448, p = 0.506) on snail biomass.
Discussion
Several brown seaweed species are harvested commercially at large scale such as Laminaria hyperborea in Norway and Lessonia spp. in Chile. The harvest of Ascophyllum differs significantly from those, in large part due to the different physiology of those species and the harvesting techniques used. In kelps such as L. hyperborea. and Lessonia spp. the meristematic tissues are located in the transition zone between the frond and stipe (Kain and Jones 1976). Most harvesting techniques will inevitably remove the meristem, and in most cases entire individuals including holdfasts are removed during harvest (Vea and Ask 2011; Vásquez et al. 2012) and recovery occurs through growth of smaller individuals left behind after harvest (Steen et al. 2016). Ascophyllum is different in that regard as new branches can be formed along shoots in lateral pits (Åberg 1996), and its recovery and growth are not dependent on an apical meristem. While this favours a rapid recovery of biomass after harvesting, it leads to a potential for the harvest to modify the morphology of clumps. The similar clump morphology and vertical biomass distribution within clumps found within harvested and control sites indicate that continual harvesting in New Brunswick over the last 20 years has not altered clump morphology. Furthermore, current morphology is similar to what has been observed prior to the onset of large-scale commercial harvesting of Ascophyllum in New Brunswick (Figs. S2 and S3); mid-biomass height ranged from 10 cm in the 0-40 cm size class to 47–55 cm in the 110–130 cm size class. Those values are similar to those observed ~ 20 years later in 2019 (12.8 and 58.0 cm for the 0–40 cm and 110–130 cm size classes respectively averaged across sites) further supporting the findings that the harvest has not modified the morphology of clumps in New Brunswick.
Ascophyllum can recover rapidly, often within a year, following harvest as it is conducted in Canada and the USA (Ugarte et al. 2006; Johnston et al. 2023). Recovery is generally faster for biomass than for height; Ascophyllum is a productive seaweed with an average 54% annual biomass turnover (Vadas et al. 2004), but with a more limited rate of elongation (10 – 20 cm y−1, Kay et al. 2016b). Although, previous estimates of growth and productivity have focused on growth in the apical region of the shoots, often overlooking the growth in older tissues, suggesting that productivity may be even greater than previously estimated (Lauzon-Guay et al. 2022). Under the current harvest management in New Brunswick, this rapid recovery after harvest has translated into no long-term (20 + years) impact of the harvest on Ascophyllum biomass or height (Lauzon-Guay et al. 2021). Similarly, there is generally no difference between harvested and control sites in the vertical distribution of the biomass and shoots along clumps. In one region, a non statistically significant trend suggest that clumps appear to be marginally heavier and have more branching lower down at the control than at the harvested site. This is the opposite of what we could expect if the harvest had an impact on clump morphology. The harvest targets the top of the clumps and we could expect that clumps would become bottom-heavy at harvested sites (i.e. more shoots and more biomass in the lower portion of the clump). Increased lateral shoots can occur with intensive cutting, where all shoots are cut within 10–15 cm of the base (Boaden and Dring 1980) and harvesting can lead to an increase growth in suppressed shoots at the base of larger clumps (Ugarte et al. 2006). Harvesting opens the canopy, presumably allowing more light to reach previously shaded shoots, not only near the holdfast but throughout the clump, resulting in faster overall growth. This may explain the greater circumference of clumps found at some harvested sites compared to control sites.
In this study the harvest did not cause clumps to become shorter, or bottom-heavy compared to unharvested clumps. This finding contradicts the assumption that the harvest would cause Ascophyllum beds to transform from “underwater forest… [to] …much shorter rockweed bush” (Seeley and Schlesinger 2012). While very intensive harvesting (60–80% of biomass removal) can alter population structure of Ascophyllum, it is resistant to long-term impact of present commercial harvesting techniques and regulations (Sharp and Pringle 1990; Lauzon-Guay et al. 2021). Two important aspects need to be considered when evaluating studies looking at the impact of Ascophyllum harvesting: the levels of the harvesting treatments, and the scale at which impacts are measured. Studies can be divided into two groups, those that use commercial harvest methods to create the harvesting treatments (e.g., Ugarte et al. 2006; Johnston et al. 2023) and those that manually apply a particular aspect of the regulation (e.g., cutting all clumps at the minimum allowed cutting heigh levels; Fegley 2001; Phillippi et al. 2014). While the latter provides more control and less variability on the treatment levels, it does not capture the heterogeneity of the commercial harvest. This explains in part why those two types of studies have led to different conclusions as to the impact of the harvest. Similarly, studies focusing on small scale impacts that follow the fate of cut individuals typically find greater and longer lasting impacts than those looking at the bed-scale impact of the harvest. The relevant scale depends on the question asked; following a tree stump would not provide valuable insights on the recovery of a forest. Similarly, the overall structure and morphology of clumps within a bed are more relevant to the associated communities than the fate of individual fronds or shoots after being cut.
To take an ecosystem-based management approach to the Ascophyllum harvest, not only the impact on the target species itself but also a broader assessment of the impact on other components of the ecosystem is required (Lotze et al. 2019). The most prevalent species found within Ascophyllum beds at low tide in the region are littorinid snails (mainly L. littorea and L. obtusata, (Adey and Hayek 2005) along with the invasive green crab (Carcinus maenas). Littorinid snails are also the main macrofauna bycatch associated with the harvest of Ascophyllum at a rate of 1.32 kg of littorinids per tonne of Ascophyllum harvested (Ugarte et al. 2010). Using the 17% exploitation rate allowed in New Brunswick and an average Ascophyllum biomass of 10.8 kg m−2 (Lauzon-Guay et al. 2021), we can estimate that 2.4 g m−2 of littorinids are removed during the harvest. Using the range of 8 to 19 g (0.06 m)−2 of littorinids observed in this study, we can estimate that between 0.8 and 1.6% of the littorinids are removed as bycatch during the harvest. This low removal rate combined with the limited impact of the harvest on the biomass, height, and morphology of Ascophyllum may explain the absence of a significant effect of harvesting on the abundance of the most abundant group of species found in Ascophyllum beds. Furthermore, by maintaining its height, biomass, and morphology, it is likely that Ascophyllum will be able to maintain its functions such as modulating understory temperature during low tide (Watt and Scrosati 2013) that are important to the assemblage of species occupying it. Those assemblages vary at local and regional scales, as do bed characteristics (Kay et al. 2016a) making it difficult to isolate the role of morphology versus that of local environmental condition on community composition. This is particularly complex because specific Ascophyllum bed characteristics will be of particular importance to different species. Shore birds tend to occupy shores with wider and thicker (measured at low tide) Ascophyllum beds, while common eiders (Somateria mollissima) are more influenced by the understorey species (Johnston et al. 2020). While we did not measure algal depth is this study, the general absence of significant difference in canopy height, biomass (Lauzon-Guay et al. 2021) and clump morphology suggest that algal depth would be maintained despite harvesting. Various fish species (e.g., Tautogolabrus adspersus, Myoxocephalus aeneus, Pollachius vires, Pseudopleuronectes americanus) are known to visit Ascophyllum beds at high tide and while the impact of complete Ascophyllum removal is equivocal (Black and Miller 1991, 1994; Rangeley 1994), normal harvesting activities do not appear to have a detectable impact on the abundance of these species (Van Guelpen and Pohle 2014). Specific information on which particular morphological characteristics beside the provision of cover and harbouring prey species are important to different fish species is not available. To better assess the morphological requirements of various species, experimental manipulations of Ascophyllum morphology in field condition could be maintained over time and changes in assemblages observed. When this has been done in relation to Ascophyllum harvesting, impacts on specific species were often small or short-lived (Fegley 2001; Trott and Larsen 2012; Phillippi et al. 2014) partially due to the rapid recovery of Ascophyllum.
The harvest of Ascophyllum is done differently depending on the region where it is harvested. In Canada, the boat and rake harvesting method has been almost exclusively used for the last 25 years (Lauzon-Guay et al. 2021). The results presented in this study relate to the impact of that harvesting technique. In Maine, a mix of boat and rake and rotating-blade type mechanical harvesters are used. Norway also harvests Ascophyllum using this type of mechanical harvester. In Iceland, reciprocating-blade type mechanical harvesters are used exclusively. This type of mechanical harvester is also used on the Isle of Lewis in the Outer Hebrides of Scotland. Further south along the Outer Hebrides, a mix of boat and rake and traditional hand harvesting on foot at low tide is used. These two methods are also used by Ascophyllum harvesters in Ireland (Mac Monagail and Morrison 2020). Typically, the traditional hand harvesters will cut Ascophyllum near the holdfast and remove a higher proportion of the standing biomass (80–90%) over a relatively small area (10-20 m) during a low tide. Mechanical harvesters tend to cut Ascophyllum higher up and remove 40–60% of the biomass over a larger area. The boat and rake harvesters generally remove less than 50% of the biomass within a harvested patch and leave a wider range of Ascophyllum lengths behind over a section of coastline (50-100 m wide). The boats are drifting as the harvest is being conducted and the action of the rake, moving diagonally through the water column, does not create a uniform cutting height (Ugarte et al. 2006). Indeed, the largest changes observed immediately after harvest within harvested patches is in relation to clump biomass rather than height or shoot density (Ugarte et al. 2006). The same cannot be said of other harvesting techniques and whether the clumps will regain their prior morphology will need to be resolved.
The harvesting of Ascophyllum as practiced under the current management regulations has very limited impact on the overall characteristics of Ascophyllum beds in North America (Lauzon-Guay et al. 2021, Johnston et al. 2023, this study). The current management framework in Canada was developed over several decades (Ugarte and Sharp 2001) and is still being actively amended by provincial authorities, in concertation with industry, to modify exploitation rates and harvesting methods as new scientific information become available. It provides a good example of adaptative governance relying on scientific knowledge, local government participation and legal adaptative capacity (Greenhill et al. 2021). From an open harvest with several companies competing for the same resource and few regulations, to the current model with sectors assigned to individual harvesting companies and regulation on minimum cutting height, holdfast removal, exploitation rate, and cutting methods, the harvest of Ascophyllum in the region has matured into what can be described as an example of sustainable management. The level of harvest is sustainable over time and discernable impacts are rare and of short duration.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Åberg P (1989) Distinguishing between genetic individuals in Ascophyllum populations on the Swedish west coast. Br Phycol J 24:183–190
Åberg P (1996) Patterns of reproductive effort in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 138:199–207
Adey WH, Hayek L-AC (2005) The biogeographic structure of the western North Atlantic rocky intertidal. Cryptogam Algol 26:35–66
Baardseth E (1955) Regrowth of Ascophyllum nodosum after harvesting. Institute for Industrial Research and Standards, Dublin 63 p
Bates CR (2009) Host taxonomic relatedness and functional-group affiliation as predictors of seaweed-invertebrate epifaunal associations. Mar Ecol Prog Ser 387:125–136
Black R, Miller R (1994) The effects of seaweed harvesting on fishes: a response. Environ Biol Fish 39:325–328
Black R, Miller R (1991) Use of the intertidal zone by fish in Nova Scotia. Environ Biol Fish 31:109–121
Boaden P, Dring M (1980) A quantitative evaluation of the effects of Ascophyllum harvesting on the littoral ecosystem. Helgol Meeresunters 33:700–710
Catalán AM, López DN, Fica‐Rojas E, Broitman BR, Valdivia N, Scrosati RA (2023) Foundation species canopies affect understory beta diversity differently depending on species mobility. Ecology 104:e3999
Chemello R, Milazzo M (2002) Effect of algal architecture on associated fauna: Some evidence from phytal molluscs. Mar Biol 140:981–990
Eger AM, Marzinelli EM, Beas-Luna R et al (2023) The value of ecosystem services in global marine kelp forests. Nat Commun 14:1894
FAO (2021) FAO Yearbook. Fishery and Aquaculture Statistics 2019. Food and Agriculture Organization of the United Nations, Rome. http://www.fao.org/fishery/statistics/
Fegley J (2001) Ecological implications of rockweed, Ascophyllum nodosum (L.) Le Jolis, harvesting. Thesis, University of Maine
Gan SX, Tay YC, Huang D (2019) Effects of macroalgal morphology on marine epifaunal diversity. J Mar Biol Assoc UK 99:1697–1707
Greenhill L, Sundnes F, Karlsson M (2021) Towards sustainable management of kelp forests: An analysis of adaptive governance in developing regimes for wild kelp harvesting in Scotland and Norway. Ocean Coast Manag 212:105816
Guiry MD, Morrison L (2013) The sustainable harvesting of Ascophyllum nodosum (Fucaceae, Phaeophyceae) in Ireland, with notes on the collection and use of some other brown algae. J Appl Phycol 25:1823–1830
Johnston E, Klemmer A, Blomberg E, Baron A, Watson VK, Tudor L, Weich LJ, Olsen BJ (2020) Macroalgae composition alters occupancy of multiple bird guilds in rocky intertidal communities. Mar Ecol Prog Ser 659:29–47
Johnston EM, Mittelstaedt HN, Braun LA, Muhlin JF, Olsen BJ, Webber HM, Klemmer AJ (2023) Bed-scale impact and recovery of a commercially important intertidal seaweed. J Exp Mar Biol Ecol 561:151869
Kay LM, Eddy TD, Schmidt AL, Lotze HK (2016a) Regional differences and linkage between canopy structure and community composition of rockweed habitats in Atlantic Canada. Mar Biol 163:251
Kay LM, Schmidt AL, Wilson KL, Lotze HK (2016b) Interactive effects of increasing temperature and nutrient loading on the habitat-forming rockweed Ascophyllum nodosum. Aquat Bot 133:70–78
Kain JM, Jones NS (1976) The biology of Laminaria hyperborea IX. Growth pattern of fronds. J Mar Biol Assoc UK 56:603–628
Lauzon-Guay JS, Feibel AI, Gibson M, Mac Monagail M, Morse BL, Robertson CA, Ugarte RA (2022) A novel approach reveals underestimation of productivity in the globally important macroalga, Ascophyllum nodosum. Mar Biol 169:143
Lauzon-Guay J-S, Ugarte RA, Morse BL, Robertson CA (2021) Biomass and height of Ascophyllum nodosum after two decades of continuous commercial harvesting in eastern Canada. J Appl Phycol 33:1695–1708
Lazo ML, Chapman A (1998) Components of crowding in a modular seaweed: sorting through the contradictions. Mar Ecol Prog Ser 174:257–267
Lemay MA, Chen MY, Mazel F, Hind KR, Starko S, Keeling PJ, Martone PT, Parfrey LW (2021) Morphological complexity affects the diversity of marine microbiomes. ISME J 15:1372–1386
Lotze HK, Milewski I, Fast J, Kay L, Worm B (2019) Ecosystem-based management of seaweed harvesting. Bot Mar 62:395–409
Mac Monagail M, Morrison L (2020) The seaweed resources of Ireland: a twenty-first century perspective. J Appl Phycol 32:1287–1300
Morais T, Inácio A, Coutinho T, Ministro M, Cotas J, Pereira L, Bahcevandziev K (2020) Seaweed potential in the animal feed: A review. J Mar Sci Eng 8:559
Norderhaug KM, Filbee-Dexter K, Freitas C, Birkely S-R, Christensen L, Mellerud I, Thormar J, van Son T, Moy F, Vasquez Alonso M, Steen H (2020) Ecosystem-level effects of large-scale disturbance in kelp forests. Mar Ecol Prog Ser 656:163–180
Pereira L, Morrison L, Shukla P, Critchley A (2020) A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J Appl Phycol 32:3561–3584
Phillippi A, Tran K, Perna A (2014) Does intertidal canopy removal of Ascophyllum nodosum alter the community structure beneath? J Exp Mar Biol Ecol 461:53–60
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rangeley RW (1994) The effects of seaweed harvesting on fishes: a critique. Environ Biol Fish 39:319–323
Rebours C, Marinho-Soriano E, Zertuche-González JA, Hayashi L, Vásquez JA, Kradolfer P, Soriano G, Ugarte R, Abreu MH, Bay-Larsen I, Hovelsrud G, Rødven R, Robledo D (2014) Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J Appl Phycol 26:1939–1951
Roberts DA, Poore AGB (2006) Habitat configuration affects colonisation of epifauna in a marine algal bed. Biol Conserv 127:18–26
Scrosati RA, Catalán AM, Valdivia N (2021) Macroalgal canopies reduce beta diversity in intertidal communities. Bot Mar 64:419–425
Seeley RH, Schlesinger WH (2012) Sustainable seaweed cutting? The rockweed (Ascophyllum nodosum) industry of Maine and the Maritime Provinces. Ann N Y Acad Sci 1249:84–103
Sharp GJ, Pringle JD (1990) Ecological impact of marine plant harvesting in the northwest Atlantic: a review. Hydrobiologia 204–205:17–24
Shukla PS, Mantin EG, Adil M, Bajpai S, Critchley AT, Prithiviraj B (2019) Ascophyllum nodosum-based biostimulants : Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:655
Steen H, Moy FE, Bodvin T, Husa V (2016) Regrowth after kelp harvesting in Nord-Trøndelag, Norway. ICES J Mar Sci 73:2708–2720
Steneck R, Johnson R (2014) Kelp forests: dynamic patterns, processes, and feedbacks. In: Bertness M, Bruno J, Siliman B, Stachowicz J (eds) Marine Community Ecology and Conservation. Sinauer Associates Inc, Massachussetts, pp 315–336
Torres AC, Veiga P, Rubal M, Sousa-Pinto I (2015) The role of annual macroalgal morphology in driving its epifaunal assemblages. J Exp Mar Biol Ecol 464:96–106
Trott TJ, Larsen PF (2010) Evaluation of short-term changes in rockweed (Ascophyllum nodosum) and associated epifaunal communities following cutter rake harvesting in Maine. Report to Maine Department of Marine Resources
Ugarte R, Bartlett C, Perry L (2010) A preliminary study to monitor periwinkle by-catch and incidence of holdfasts in harvested rockweed. Report to Maine Department of Marine Resources. 13 pp
Ugarte RA, Sharp G (2001) A new approach to seaweed management in Eastern Canada: The case of Ascophyllum nodosum. Cah Biol Mar 42:63–70
Ugarte RA, Sharp GJ, Moore B (2006) Changes in the brown seaweed Ascophyllum nodosum (L.) Le Jol. plant morphology and biomass produced by cutter rake harvests in southern New Brunswick, Canada. J Appl Phycol 18:351–359
Vadas RL, Wright WA, Beal BF (2004) Biomass and productivity of intertidal rockweeds (Ascophyllum nodosum Le Jolis) in Cobscook Bay. Northeast Nat 11:123–142
Van Guelpen L, Pohle G (2014) Short- and long-term impact of rockweed harvesting on the intertidal fish community in southwest New Brunswick. Huntsman Marine Science Center. Final Report to the New Brunswick Wildlife Trust Fund. Project F303–052. 29 p
Vásquez JA (2008) Production, use and fate of Chilean brown seaweeds: re-sources for a sustainable fishery. J Appl Phycol 20:457–467
Vásquez JA, Piaget N, Alonso Vega JM (2012) The Lessonia nigrescens fishery in northern Chile: “how you harvest is more important than how much you harvest.” J Appl Phycol 24:417–426
Vea J, Ask E (2011) creating a sustainable commercial harvest of Laminaria hyperborea, in Norway. J Appl Phycol 23:489–494
Watt CA, Scrosati RA (2013) Bioengineer effects on understory species richness, diversity, and composition change along an environmental stress gradient: Experimental and mensurative evidence. Estuar Coast Shelf Sci 123:10–18
White CR (2003) Allometric analysis beyond heterogeneous regression slopes: Use of the Johnson-Neyman technique in comparative biology. Physiol Biochem Zool 76:135–140
Wikström SA, Kautsky L (2004) Invasion of a habitat-forming seaweed: Effects on associated biota. Biol Invasions 6:141–150
Acknowledgements
Collette Robertson and Britton Skuse for their help with field sampling and lab processing.
Funding
This work was supported by Acadian Seaplants Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by JSLG, AIF, and BLM. Data analysis was performed by JSLG. The first draft was written by JSLG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors are employees of Acadian Seaplants Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lauzon-Guay, JS., Feibel, A.I., Morse, B.L. et al. Morphology of Ascophyllum nodosum in relation to commercial harvesting in New Brunswick, Canada. J Appl Phycol 35, 2371–2381 (2023). https://doi.org/10.1007/s10811-023-03028-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03028-6