Abstract
Ascophyllum nodosum (L.) Le Jolis (Fucales, Fucaceae) is a modular intertidal brown alga that has the particularity of forming an air bladder once a year at the apical tip of the growing shoots. This characteristic provides a means for aging and estimating the growth of individuals. While it has long been recognized that growth can occur in older parts of the frond, this has not been properly assessed until now and has largely been overlooked when calculating the productivity of the species. Recent studies have suggested that the growth and elongation of older segments is minimal and thus has been used to infer past environmental conditions. Here we assessed the length and mass of successive internodal segments from 25 sites spread over both sides of the North Atlantic, covering a wide portion of the distribution of the species. By calculating the ratio of the mass and length of a segment divided by the segment produced the following year, we established that internodal segments continue accumulating mass for 1–5 years and increase in length for 1–3 years at most sites. Segments can almost triple their mass during their second year and more than double their length. These results indicate that previous productivity and growth estimates for A. nodosum based on apical growth alone greatly underestimate the true productivity of the species and its role in coastal carbon cycling. Furthermore, because they grow over several years, internodal segments should not be used to infer past environmental conditions or to reconstruct growth patterns over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ecological importance of macroalgal beds has long been understood, providing many crucial ecosystem services and functions. Their tridimensional structure provides habitat (Teagle et al. 2017) and alters flow dynamics (Eckman et al. 1989). They also play an important role in nutrient cycling (Bennett et al. 2016), primary production (Duarte et al. 2022), and their detrital production is an important food subsidy in deeper areas denuded of vegetation (Krumhansl and Scheibling 2012). In recent years, the contribution of wild and cultivated seaweed to carbon absorption and sequestration has gained increased attention (Krause-Jensen and Duarte 2016; Duarte et al. 2022; Sato et al. 2022). Often overlooked in the past, they are now recognized as an important component of the global carbon cycle (Duarte et al. 2022) and constitute the largest vegetated coastal biome on the planet (Pessarrodona et al. 2022a). It has been recently estimated that macroalgae occupy over 6 million km2 of the ocean floor, 13,000 km2 of which are in the intertidal zone, translating into a Net Primary Productivity (NPP) of 8 million t C/y in the intertidal zone alone (Duarte et al. 2022). Estimates of the growth (increase in mass or length at the individual level) and productivity (increase in biomass per unit of area or biomass) of seaweeds can be difficult to obtain. For example, in situ measurements often rely either on changes in standing stock (e.g., Brinkhuis 1977), by marking individuals and measuring them over time (e.g., Chapman and Craigie 1977), or can be assessed directly in some species that can be aged or where structures are shed every year (e.g., Laminaria hyperborea; Sjøtun and Frederiksen 1995). Furthermore, productivity estimates can vary significantly between locations (see Pessarrodona et al. 2022b for a review), requiring sampling at multiple sites to obtain meaningful estimates.
Ascophyllum nodosum (L.) Le Jolis is a perennial brown alga that dominates the sheltered rocky intertidal in most of its distribution on both sides of the North Atlantic (Pereira et al. 2020). It is a modular species where fronds grow from a common holdfast (Lazo and Chapman 1998). An air bladder is formed annually in the spring along each shoot (Marbà et al. 2017), although the formation of the first air bladder is indeterminate and can take several years (Keser and Larson 1984; R. Ugarte pers obs). Therefore, only the minimum age of a plant can be ascertained, which does not generally exceed 17–20 years (Marbà et al. 2017, J-S Lauzon-Guay, pers obs).
Shoots can divide during the year (but this does not always occur) into two or more branches (up to 11; B Morse, pers obs; Fig. 1), and lateral shoots will also form along the sides of the main shoot (David 1943). Besides aging shoots, air bladders have also been used to calculate internodal lengths (distance between successive air bladders) to retrospectively quantify the growth of A. nodosum and link it with past environmental conditions (Marbà et al. 2017). As intertidal species are particularly sensitive to changing temperature (Helmuth et al. 2006), A. nodosum, with its distinctive annual air bladders, may be a good bioindicator of climate change (Marbà et al. 2017). These yearly air bladders have also been used to calculate yearly growth and productivity estimates for the species (Baardseth 1970; Cousens 1984; Vadas et al. 2004). Ascophyllum nodosum is commercially harvested on both sides of the North Atlantic (Mac Monagail et al. 2017), and quotas are often determined based on productivity estimates of the species to ensure recovery (Ugarte and Sharp 2001; Lauzon-Guay et al. 2021).
The extent to which thickening and elongation occur in older parts of the shoots is unclear. By comparing the elongation in the apical versus distal half of a shoot, David (1943) concluded that “growth is apical” and “very slight in proximal regions”. Nonetheless, Baardseth (1955) indicated that growth would continue over time in older segments and estimated productivity by dividing shoots into internodal segments of different ages. Meanwhile, Aberg (1996) suggests that “the growth is more pronounced [“confined largely” in Cousens 1986] above the two most recently produced bladders and generally there is no secondary thickening (Burrows 1947 in Cousens 1986).” This has led authors, in more recent years, to only use the last formed (most apical) segments to calculate productivity (Cousens 1984; Vadas et al. 2004). This notion was supported by Eckersley and Garbary (2007), who found no elongation in older segments in three A. nodosum populations in Nova Scotia.
Meanwhile, a casual observer will notice that older parts of a shoot are often much thicker than the tips. Similarly, older air bladders can be much larger and thicker than newly formed ones (Garbary et al. 2006). These observations partially contradict some published findings and may be due to differences in growth between populations or regions. To evaluate whether growth in terms of shoot elongation and mass is occurring in older shoot segments, we sampled shoots from 25 sites spanning 14° of latitude from both sides of the North Atlantic and measured the length and mass of individual internodal segments on unbroken shoots.
Materials and methods
Field sampling
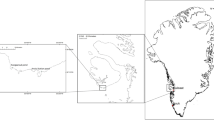
The study area for this project spanned both sides of the North Atlantic, with samples collected from 25 sites divided between the coasts of the Outer Hebrides in Scotland, western Ireland, eastern Canada, and Maine, USA (Fig. 2). Sampling took place at 23 of the sites in April 2021, prior to gamete release and the loss of receptacles but after the formation of the annual air bladder at the apical tip of the shoots. Except for Scotland and Tor Bay in Nova Scotia, all sites are in areas where commercial harvesting takes place. Because the harvest can increase productivity, two extra sites were sampled in Scotland in early May 2022 (Lochmaddy and Grimsay); both sites had been commercially harvested 4–5 years earlier. All sites were sheltered to semi-sheltered and mostly consisted of large beds (> 100 m along the shore) of dense A. nodosum interspersed with Fucus vesiculosus. The bed at Tangier was noticeably smaller (30–40 m in length along the shore) with a patchy cover of A. nodosum.
Map of Eastern North America showing study sites in Nova Scotia, New Brunswick, and Maine with inset (1) showing study sites within Cobscook Bay, Maine and New Brunswick. Map of Ireland and the United Kingdom showing regions in the Outer Hebrides, Scotland (2) and Ireland (3) where study sites are located
At each site, sampling was conducted during a 4 h window around low tide. A 30 m transect line was deployed at the mid-intertidal height of the A. nodosum bed, parallel to the shore. Twenty 10 × 10 cm (0.01 m2) quadrats were randomly positioned along the length of the transect. Within each quadrat, individual A. nodosum clumps (all shoots originating from a common holdfast) were zip tied directly above the holdfast, then collected with holdfasts intact. If no A. nodosum was present in a quadrat, the quadrat was randomly repositioned. The number of reposition attempts was recorded. Specimens were transported in plastic bags and stored at 4° C prior to processing.
Other data sources
Previously reported length and mass data were extracted from figures in Eckersley and Garbary (2007) and Marbà et al. (2017) using the Figure Calibration plugin in ImageJ. The Greenland Ecosystem Monitoring Project (GEM) has been collecting the length and mass of the first three internodal segments (tip and last 2 years of growth) from three sites in Kobbefjord in West Greenland since 2012. We retrieved these data from the GEM website (https://doi.org/10.17897/KEMY-JJ24 and https://doi.org/10.17897/4NJK-ZV13). We only used the data from the mid-intertidal sampling location from all three sites as this was the only tidal elevation available from all three sites, and it is comparable to our data.
Laboratory processing
The longest continuous and unbroken shoot from each quadrat was selected, and all lateral branches and receptacles were cut off with a knife. The shoot was blotted dry, and epiphytes were removed. The total length and number of air bladders present were recorded. Annual segments were removed by cutting below each annual air bladder. The length (to the nearest mm) of each segment was recorded with a metric ruler, and the mass (to the nearest 0.01 g) was recorded using a digital scale (various models). All segments from the same shoot were weighted consecutively within a 1-min window to ensure that no evaporation difference would affect the results.
Statistical analysis
We followed Cousens (1984) in numbering segments. The apical growth segment, S0, located from the growing tip to below the newly formed air bladder, did not represent a full year of growth and was not used in the analysis. Subsequent segments were numbered based on the years since their formation (S1: produced 1 year ago, S2: 2 years ago, etc.) until the last segment terminated at the holdfast. This last segment was not included in the analysis because the first air bladder is not always produced after 1 year (Keser and Larson 1984), and it is not possible to age that segment. To evaluate whether segments increased in mass (or length) after the formation of the new air bladder, the ratios of a segment’s mass (or length) Si divided by the segment produced the following year Si-1 was calculated for each segment, starting at S2. These ratios were calculated for each shoot, and the 95% confidence interval (1-tail) was calculated for each site for all segments with at least three replicates because not all longest shoots had the same number of segments. A confidence interval greater than one indicates that a segment increased in mass (or length) over the year.
We used the first ratio (S2:S1) to evaluate differences between regions using a two-way ANOVA with Site as a random factor nested within Region (fixed factor). Separate analyses were done for the mass and length ratios. The relationship between the mass (covariate) and length second ratio (S2:S1) between sites was evaluated using an ANCOVA with Region as a factor. All statistical analyses were done in R v4.2.0 (R Core Team 2022).
Only average values could be extracted from figures in Eckersley and Garbary (2007) and Marbà et al. (2017); thus, no statistical analysis was done. Data collected by the GEM in West Greenland were used to calculate the S2:S1 ratio using the segment collected each year, because the data were collected over several years, we also calculated the ratio of S2:S1 from consecutive years (i.e., the same segment position but not from the same shoot in consecutive years). S1 in 2012 would become S2 in 2013, S1 in 2013 would become S2 in 2014 and so on. By calculating the ratio between 2 consecutive years, we are limiting the possibility that the differences observed are due to environmental conditions.
Results
At 76% of sites (19 out of 25), segments increased significantly in length for 1–3 consecutive years after formation (Fig. 3). The increase was typically greatest for the second segment or during the second year of growth, where the length of a segment could double during its second year (Fig. 3, Lochmaddy). Except in rare cases, older growth segments (> 5 years) did not increase in length.
Ratio of the length of Segmenti: Segmenti-1 for each site within each region (rows: Maine, New Brunswick, Nova Scotia, Scotland 2021, Scotland 2022, Ireland). Closed symbols indicate that the 95% confidence interval is greater than one. Horizontal dashed line at ratio = 1 indicate no change in length between successive segments
Similarly, segments increased in mass for 1–5 consecutive years after their formation at all sites (Fig. 4). The increase in mass was greater than the increase in length during its second year; a segment could almost triple its mass. Older ratios were not statistically greater than one, indicating that no growth occurred after 6 years at any site.
Ratio of the mass of Segmenti: Segmenti-1 for each site within each region (rows: Maine, New Brunswick, Nova Scotia, Scotland 2021, Scotland 2022, Ireland). Closed symbols indicate that the 95% confidence interval is greater than one. Horizontal dashed line at ratio = 1 indicate no change in mass between successive segments
Overall, there were no statistically significant regional differences between the second ratio (S2:S1) for length (ANOVA, F (4,23.23) = 0.629, P = 0.646), and the mass ratio was marginally non-significant (ANOVA, F (4,25.28) = 2.336, P = 0.082; Fig. 5). The slopes of the linear relationships between the second ratio (S2:S1) mass and length was not statistically different between regions (ANCOVA, F (4,15) = 0.341, P = 0.846). Overall, a significant linear relationship (ANCOVA, F (1,19) = 61.304, P < 0.001) existed between the mass and length ratios for S2 between sites (Fig. 6), indicating that sites where segments increased the most in lengths are also sites where segments increased the most in mass. Region had a statistically significant effect on the intercept of this relationship (ANCOVA, F (4,19) = 4.155, P = 0.014), with sites from Scotland having a lower intercept than all others (t test, t19 = -3.929, P = 0.045). For a similar length ratio, A. nodosum from sites in Scotland had a lower mass ratio than in other regions.
Linear relationship between the average mass and length ratio for the second growth segment (S2:S1) at each site within regions. Dashed line represents the linear regression for all sites excluding Scotland (r2 = 0.61) while the dotted line represents the linear regression for sites from Scotland only (r2 = 0.95)
The data obtained from the Greenland Ecosystem Monitoring project (GEM) showed that only at the central site in 2016 was the S2:S1 length ratio from successive years below one, all other years and sites (n = 20) had a ratio above one with an overall average of 1.12 (Fig. 7a). The average ratio calculated from segments collected in the same year was identical at 1.12. At all three sites from West Greenland, the S2:S1 mass ratio based on successive years is above one for all years (Fig. 7b). The average over all years and sites is 1.61, similar to the ratio calculated using segments from the same year (S2:S1 = 1.65). Both ratios varied between years, with some years showing almost no secondary growth (e.g., 2017) while others had segments more than double in mass during their second year (Fig. 7b). Interannual variation in growth explains a large part of that variation since the S2:S1 mass ratio correlates strongly (Pearson’s correlation, r = 0.72) with the annual growth anomaly (annual growth of S1– average over all years, Fig. 8). This correlation is expected since growing conditions during the second full year of growth of a segment will directly impact the ratio.
Length (a) and mass (b) S2:S1 ratio for three sites from West Greenland. Ratios were calculated using data from consecutive years. Data from: https://doi.org/10.17897/KEMY-JJ24 and https://doi.org/10.17897/4NJK-ZV13
Correlation (r = 0.72) between the S2:S1 mass ratio calculated using segments from consecutive years from the Inner site in Greenland and the growth anomaly for the second year of growth using the average mass of the first segment in a year in relation to the average mass over all years. Data from: https://doi.org/10.17897/4NJK-ZV13
Discussion
Our results demonstrate that shoot elongation and mass growth is not restricted to the most apical segment in A. nodosum. Our study encompassed sites from both sides of the Atlantic Ocean, spanning 14° of latitude with various levels of wave exposure, substrate, and growth rates (Lauzon-Guay et al. in prep), suggesting that this phenomenon is widespread and likely applies to most A. nodosum populations. These results directly impact the study of the ecology and physiology of A. nodosum but also have implications for harvesting regulations, coastal management and the value attributed to coastal ecosystems. Most previous studies (e.g., Cousens 1984; Vadas et al. 2004) measuring the productivity or growth of A. nodosum only looked at the elongation or gain in mass of the most recently formed segment (S1). We have observed that during its second year, a segment can more than double in mass and increase in length by up to 50%. Depending on the site, a segment can keep increasing in mass and length for several years. Similarly, air bladders in A. nodosum undergo enlargement for at least 4 years (Garbary et al. 2006). Therefore, calculating productivity based on S1 alone greatly underestimates the real productivity and growth rate of the species.
Several field observations support the findings of this study. Changes in dry mass between the lowest spring value and the highest late summer value suggest an increase in biomass of up to 80% in Norway (Baardseth 1970), almost twice the productivity calculated using the most apical internodal segments in Maine (Vadas et al. 2004). Similarly, the free-living form of Ascophyllum nodosum (ecad mackaii) can multiply its mass by 18 times over a 3-year period through both apical and secondary growth (Gibb 1957). Experimental plots, where 50% of the biomass was harvested, recovered their pre-harvest biomass in a year in New Brunswick, Canada (Ugarte et al. 2006) and 2 years in Scotland (Walker 1948), faster than would be expected from the growth of the apical segment alone. This finding has significant management implications.
Ascophyllum nodosum is commercially harvested in several countries (mainly Canada, Ireland, Norway, Iceland, USA, Scotland, and France). Regulations vary between countries; for example, the maximum harvest rate in New Brunswick, Canada, is set at 17% of the standing stock (Ugarte and Sharp 2001; Lauzon-Guay et al. 2021). According to previous productivity estimates based on the most apical growth segment (Cousens 1984), it was estimated that this harvest rate represented between 15.1 and 18.7% of the annual A. nodosum production in southern New Brunswick (Ugarte et al. 2006). Only vegetative production is included in this analysis but A. nodosum can have up to 50% of its wet weight in reproductive structures in early spring prior to gamete release (Pereira et al. 2020), making the proportion of the biomass removed during the harvest an even smaller proportion of the total annual production. Based on our results, the proportion of annual production harvested is much smaller considering segments grew by over 85% of their mass during their second year on average in New Brunswick. The exact resulting increase in productivity from growth occurring in older segments cannot be directly extrapolated from the results of this study because the number of segments along a shoot will decrease from the tip to the holdfast as the shoot bifurcates. The extent of bifurcation would have to be measured at each site to estimate the productivity of a shoot. While this will be less than the observed ratios, it will nonetheless significantly contribute to the overall productivity. Even if all shoots bifurcate yearly (which is not usually the case, J-S Lauzon-Guay, pers obs), we could still expect a 42.5% (85%/2) increase in productivity based on the growth occurring during the second year in S2 alone compared to current estimates based only on S1. If we included growth of older segments, this would increase productivity, reducing further the proportion of the annual growth removed during harvest. This would also explain why continuous harvest below a 25% harvest rate has no or limited impact on the height of the A. nodosum canopy after several years (Lauzon-Guay et al. 2021). Many studies have used internodal length to estimate annual growth (MacFarlane 1933; Eckersley and Garbary 2007; Marbà et al. 2017), but as our data show, elongation during the second year can be as high as 50% of the first year’s growth. This suggests that growth estimates based on internodal distances underestimate the actual growth in length of A. nodosum.
Our findings highlight the flawed assumption that internodal distances can be used to estimate growth from past years. Because A. nodosum produces a single air bladder along a shoot each year, internodal distances have been used to estimate growth over time and link past growth to environmental conditions (e.g., Marbà et al. 2017). A necessary assumption being that no (or very little) growth occurred after a segment was produced. Our analyses indicate that this assumption is incorrect in most populations, as segments keep growing for several years, and their length likely reflects the environmental conditions over several years. This assumption casts doubts on the use of internodal distance as a proxy for past environmental conditions. Furthermore, such an analysis assumes that segment elongation is not affected by their position within the canopy. Since tip elongation is usually reduced in sub-canopy and suppressed shoots compared to canopy shoots (Vadas et al. 2004), this method will undoubtedly indicate an increase in growth over time (e.g., Marbà et al. 2017) irrespective of actual growing conditions.
Eckersley and Garbary (2007) did not detect statistically significant differences in either the length or mass between S1 and S2 at any of their three sites in Nova Scotia, Canada, despite a large sample size. This contradicts our findings, especially because we re-sampled one of their original sites (Tor Bay). A re-analysis of their data indicates that mass and length ratios for the second segment were also above one at two of their three sites (Fig. S1). While the ratios calculated from Eckersley and Garbary’s paper were lower than the ones obtained in this study (1.20 vs 1.75 for mass and 1.10 vs 1.25 for length), the differences can be explained by the timing of the sampling (Fall vs Spring). S1 and S2 had an extra spring and summer to grow when sampled by Eckersley and Garbary (2007). The reproductive season and gamete release ends in spring in A. nodosum (Aberg 1996), and shoots would have had more resources to allocate to growth over the summer. Since the difference between successive segments decreases over time, this late sampling would have decreased the differences, making it harder to detect. The different conclusions can also be attributed to different analyses. Eckersley and Garbary (2007) compared the average length (or mass) of segments within a site, not considering that segments came from the same shoots and that their lengths were most likely correlated. Conversely, we compared segments within each shoot by calculating the ratio for each shoot separately and then calculated the averages (equivalent to a paired t test).
Marking individual segments and following their length and mass over time (the latter would be impossible to do) would offer a more direct means of assessing the growth of segments over time. A certain amount of variability in interannual growth is expected, and not all the differences between segments can be attributed to continued growth over time. Nonetheless, based on measurements of S1 over two successive springs (2019 and 2020) at 31 sites in the USA and Canada, we observed an average (± SE) length ratio of 1.01 ± 0.03 (Lauzon-Guay et al., unpubl data). Therefore, we are confident that the length ratios significantly greater than one observed in this study are caused by continued growth over time rather than interannual differences in growth. Furthermore, the multi-year data collected from West Greenland allows direct comparison of a segment between 2 consecutive years. Those results concur with our dataset and indicate that segments increase in length and mass over time. These multi-year data also indicate that the exact ratio will vary over time and depends on the environmental growing conditions during both years. A higher than usual growth rate during its second full year of growth will increase the ratio compared to slow growth years. This may explain why some previous studies have observed little to no secondary growth (Eckersley and Garbary 2007).
It must be acknowledged that some growth has been expected to occur in older segments (e.g., Baardseth 1955; Keser and Larson 1984) and was accounted for in early productivity estimates. Nonetheless, in more recent years, it has been assumed to be negligible (e.g., Cousens 1984; Vadas et al. 2004), and recent field data did not provide strong support (Eckersley and Garbary 2007). Our study provides data from multiple sites across a wide geographical range and provides unambiguous results showing that significant growth occurs in older segments in A. nodosum.
Our study highlights the need for new estimates of productivity of A. nodosum to account for the growth of the entire shoot rather than based on the apical tip only as generally occurs (Cousens 1981, 1984; Vadas et al. 2004). This will aid seaweed harvesters and managers to establish appropriate harvesting rates for each population. Long-term annual harvesting below 25% of the standing stocks has not resulted in significant changes in biomass of A. nodosum in New Brunswick and Nova Scotia (Lauzon-Guay et al. 2021). However, without accurate productivity estimates, it is difficult to assess what harvesting rate should be used in other regions with different productivity levels.
Furthermore, because most previous studies have assessed the productivity of A. nodosum based on apical growth alone, its true productivity has been underestimated, and its overall role in coastal and oceanic carbon budgets has been overlooked along with that of seaweeds in general (Pessarrodona et al. 2022a). With nearly half of the earth’s NPP occurring in the oceans (Field et al. 1998) and the urgent need to better understand carbon cycling and more specifically the potential role of seaweed in carbon sequestration (Krause-Jensen et al. 2018), obtaining accurate estimates of seaweed productivity will become essential. Including biased estimates when calculating the role of seaweed in global carbon cycling may lead to an undervaluation of their true value and affect climate change policy decisions (Duarte et al. 2017; Froehlich et al. 2019). Future studies should use proper metrics to quantify productivity in this species and others to allow for informed management and policy development.
Availability of data and materials
The datasets generated during the current study are not publicly available due to commercial confidentiality reasons but are available from the corresponding author on reasonable request.
References
Aberg P (1996) Patterns of reproductive effort in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 138:199–207. https://doi.org/10.3354/meps138199
Baardseth E (1955) Regrowth of Ascophyllum nodosum after harvesting. Dublin, Institute for Industrial Research and Standards, p 63
Baardseth E (1970) Synopsis of Biological Data on knobbed wrack FAO Fisheries Synopsys, pp 38-46.
Bennett S, Wernberg T, Connell SD, Hobday AJ, Johnson CR, Poloczanska ES (2016) The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar Freshw Res 67:47–56. https://doi.org/10.1071/MF15232
Brinkhuis BH (1977) Comparisons of Salt-marsh fucoid production estimated from three different indices. J Phycol 13(4):328–335. https://doi.org/10.1111/j.1529-8817.1977.tb02936.x
Burrows EM (1947) A biological investigation of the behaviours of Ascophyllum nodosum over a period of years and an enquiry into its relations with other components of an algal flora. PhD Thesis, London University, London, UK.
Chapman ARO, Craigie JS (1977) Seasonal growth in Laminaria longicruris: Relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar Biol 40(3):197–205. https://doi.org/10.1007/bf00390875
Cousens R (1984) Estimation of annual production by the intertidal brown alga Ascophyllum nodosum (L.) Le Jolis. Bot Mar. https://doi.org/10.1515/botm.1984.27.5.217
Cousens R (1986) Quantitative reproduction and reproductive effort by stands of the brown alga Ascophyllum nodosum (L.) Le Jolis in south-eastern Canada. EstuarCoast Shelf Sci 22:495–507. https://doi.org/10.1016/0272-7714(86)90071-5
Cousens R (1981) Variation in annual production by Ascophyllum nodosum (L.) Le Jolis with degree of exposure to wave action. In: International Seaweed Symposium (Xth). De Gruyter, p 253–258
David HM (1943) Studies in the autecology of Ascophyllum nodosum Le Jol. J Ecol 31:178–198. https://doi.org/10.2307/2256547
Duarte CM, Wu J, Xiao X, Bruhn A, Krause-Jensen D (2017) Can seaweed farming play a role in climate change mitigation and adaptation? Front Mar Sci 4:100. https://doi.org/10.3389/fmars.2017.00100
Duarte CM, Gattuso J, Hancke K, Gundersen H, Filbee-Dexter K, Pedersen MF, Middelburg JJ, Burrows MT, Krumhansl KA, Wernberg T et al (2022) Global estimates of the extent and production of Macroalgal Forests. Glob Ecol Biogeogr 31:1422–1439. https://doi.org/10.1111/geb.13515
Eckersley LK, Garbary DJ (2007) Developmental and environmental sources of variation on annual growth increments of Ascophyllum nodosum (Phaeophyceae). Algae 22:107–116. https://doi.org/10.4490/algae.2007.22.2.107
Eckman JE, Duggins DO, Sewell AT (1989) Ecology of under story kelp environments I Effects of kelps on flow and particle transport near the bottom. J Exp Mar Biol Ecol 129(2):173–187. https://doi.org/10.1016/0022-0981(89)90055-5
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281:237–240. https://doi.org/10.1126/science.281.5374.237
Froehlich HE, Afflerbach JC, Frazier M, Halpern BS (2019) Blue growth potential to mitigate climate change through seaweed offsetting. Curr Bio 29(18):3087–3093. https://doi.org/10.1016/j.cub.2019.07.041
Garbary DJ, Brackenbury A, McLean AM, Morrison D (2006) Structure and development of air bladders in Fucus and Ascophyllum (Fucales, Phaeophyceae). Phycologia 45(5):557–566. https://doi.org/10.2216/05-62.1
Gibb DC (1957) The free-living forms of Ascophyllum nodosum (L.) Le Jol. J Ecol 45:49–83
Helmuth B, Mieszkowska N, Moore P, Hawkins SJ (2006) Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu Rev Ecol Evol 37:373–404. https://doi.org/10.1146/annurev.ecolsys.37.091305.110149
Keser M, Larson BR (1984) Colonization and growth of Ascophyllum nodosum (Phaeophyta) in Maine. J Phycol 20:83–87. https://doi.org/10.1111/j.0022-3646.1984.00083.x
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–774. https://doi.org/10.1038/ngeo2790
Krause-Jensen D, Lavery P, Serrano O, Marbà N, Masque P, Duarte CM (2018) Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol Lett 14:20180236. https://doi.org/10.1098/rsbl.2018.0236
Krumhansl KA, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302. https://doi.org/10.3354/meps09940
Lauzon-Guay J-S, Ugarte RA, Morse BL, Robertson CA (2021) Biomass and height of Ascophyllum nodosum after two decades of continuous commercial harvesting in eastern Canada. J Appl Phycol 33:1695–1708. https://doi.org/10.1007/s10811-021-02427-x
Lazo ML, Chapman A (1998) Components of crowding in a modular seaweed: sorting through the contradictions. Mar Ecol Prog Ser 174:257–267. https://doi.org/10.3354/meps174257
Mac Monagail M, Cornish L, Morrison L, Araújo R, Critchley AT (2017) Sustainable harvesting of wild seaweed resources. Eur J Phycol 52:371–390. https://doi.org/10.1080/09670262.2017.1365273
MacFarlane C (1933) Observations on the annual growth of Ascophyllum nodosum. Proc N S Inst Sci 18(2):27–33
Marbà N, Krause-Jensen D, Olesen B, Christensen PB, Merzouk A, Rodrigues J, Wegeberg S, Wilce RT (2017) Climate change stimulates the growth of the intertidal macroalgae Ascophyllum nodosum near the northern distribution limit. Ambio 46:119–131. https://doi.org/10.1007/s13280-016-0873-7
Pereira L, Morrison L, Shukla P, Critchley A (2020) A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J Appl Phycol 32:3561–3584. https://doi.org/10.1007/s10811-020-02246-6
Pessarrodona A, Asis J, Filbee-Dexter K, Burrows MT, Gattuso J-P, Duarte CM, Krause-Jensen D, Moore PJ, Smale DA, Wernberg T (2022a) Global seaweed productivity. Sci Adv. https://doi.org/10.1126/sciadv.abn2465
Pessarrodona A, Filbee-Dexter K, Krumhansl KA, Moore PJ (2022b) Wernberg T (2022b) A global dataset of seaweed net primary productivity. Sci Data 9:484. https://doi.org/10.1038/s41597-022-01554-5
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Sato Y, Nishihara GN, Tanaka A, Belleza DFC, Kawate A, Inoue Y, Hinode K, Matsuda Y, Tanimae S, Tozaki K, Terada R, Endo H (2022) Variability in the Net Ecosystem Productivity (NEP) of seaweed farms. Front Mar Sci. https://doi.org/10.3389/fmars.2022.861932
Sjøtun K, Frederiksen S (1995) Growth allocation in Laminaria hyperborea (Laminariales, Phaeophyceae) in relation to age and wave exposure. Mar Ecol Prog Ser 126:213–222. https://doi.org/10.3354/meps126213
Teagle H, Hawkins SJ, Moore PJ, Smale DA (2017) The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J Exp Mar Biol Ecol 492:81–98. https://doi.org/10.1016/j.jembe.2017.01.017
Ugarte RA, Sharp G (2001) A new approach to seaweed management in Eastern Canada: The case of Ascophyllum nodosum. Cah Biol Mar 42:63–70
Ugarte RA, Sharp GJ, Moore B (2006) Changes in the brown seaweed Ascophyllum nodosum (L.) Le Jol. plant morphology and biomass produced by cutter rake harvests in southern New Brunswick. Canada J Appl Phycol 18:351–359. https://doi.org/10.1007/s10811-006-9044-8
Vadas RL, Wright WA, Beal BF (2004) Biomass and productivity of intertidal rockweeds (Ascophyllum nodosum Le jolis) in Cobscook Bay. Northeast Nat 11:123–142. http://www.jstor.org/stable/60225652
Walker FT (1948) Regrowth of Ascophyllum nodosum after cutting. Scottish Seaweed Research Association Report no. 108. Institute of Seaweed Research. Midlothian. 7pp
Funding
This work was supported by Acadian Seaplants Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by JSLG, AIF, MG, MMM, BM and CAR. Data analysis was performed by JSLG. The first draft was written by JSLG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of Acadian Seaplants Ltd or one of its subsidiaries.
Ethical approval
This study does not include animal or human subjects and did not require ethics approval.
Additional information
Responsible Editor: M. Roleda.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lauzon-Guay, JS., Feibel, A.I., Gibson, M. et al. A novel approach reveals underestimation of productivity in the globally important macroalga, Ascophyllum nodosum. Mar Biol 169, 143 (2022). https://doi.org/10.1007/s00227-022-04133-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04133-9