Abstract
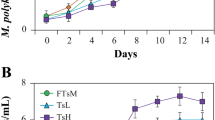
A huge interest in CO2-tolerant microalgae is fueled by development of CO2-biomitigation methods based on intensive cultivation of microalgae. Still, the mechanisms of CO2-tolerance are scarcely investigated. Previously, we described a symbiotic Desmodesmus sp. IPPAS S-2014 from a White Sea hydroid tolerant to extremely high (20–100%) CO2 levels. In the present work, we compared its ultrastructural and physiological responses to those of a novel free-living White Sea strain of Tetradesmus obliquus IPPAS S-2023 characterized in the companion paper. The strain S-2023 is closely related to Desmodesmus sp. IPPAS S-2014 but lacks its tolerance to extremely high CO2 (it is unable to survive at 100% CO2 and exhibits a reduced-growth phenotype when sparged with 20% CO2: air mixture). We compared the responses of the cell organization and photosynthetic activity to 20% CO2 in the tolerant and the intolerant White Sea chlorophytes using chlorophyll fluorescence measurements and ultrastructural analysis (transmission electron microscopy). The features peculiar to the CO2-intolerant chlorophyte include (i) inability to maintain pH homeostasis, (ii) a steady decline in the photosynthetic activity of the cells, (iii) a reduction of the photosynthetic membranes, and (iv) delayed accumulation of starch (starch grains) and its subsequent conversion to reserve lipids (oil bodies). Nitrogen starvation enhances the effects of high-CO2 stress in the CO2-intolerant microalga. The results of this work are discussed in the context of selection of tolerant algal strains for CO2 biomitigation applications.







Similar content being viewed by others
Notes
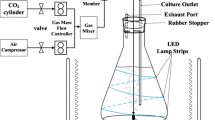
Here and below, volumetric concentrations of CO2 are specified.
References
Baulina O, Gorelova O, Solovchenko A, Chivkunova O, Semenova L, Selyakh I, Scherbakov P, Burakova O, Lobakova E (2016) Diversity of the nitrogen starvation responses in subarctic Desmodesmus sp. (Chlorophyceae) strains isolated from symbioses with invertebrates. FEMS Microbiol Ecol 92:fiw031
Carreres BM, de Jaeger L, Springer J, Barbosa MJ, Breuer G, Van den End EJ, Kleinegris DMM, Schäffers I, Wolbert EJH, Zhang H, Lamers PP, Draaisma RB, Martins dos Santos VAP, Wijffels RH, Eggink G, Schaap PJ, Martens DE (2017) Draft genome sequence of the oleaginous green alga Tetradesmus obliquus UTEX 393. Genome Announc 5. doi:https://doi.org/10.1128/genomeA.01449-16
Cheah WY, Show PL, Chang JS, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201
Demidov E, Iwasaki I, Sato N (2000) Short-term responses of photosynthetic reactions to extremely high-CO2 stress in a “high-CO2 tolerant” green alga Chlorococcum littorale and an intolerant green alga Stichococcus bacillaris. Russ J Plant Physiol 47:622–631
Gao K, Helbling EW, Häder DP, Hutchins DA (2012) Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Mar Ecol Prog Ser 470:167–189
Giordano M, Beardall J, Raven J (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gorelova O, Kosevich I, Baulina O, Fedorenko T, Torshkhoeva A, Lobakova E (2009) Associations between the White Sea invertebrates and oxygen-evolving phototrophic microorganisms. Moscow Univ Biol Sci Bull 64:16–22
Gorelova O, Baulina O, Solovchenko A, Chekanov K, Chivkunova O, Fedorenko T, Lobakova E (2015a) Similarity and diversity of the Desmodesmus spp. microalgae isolated from associations with White Sea invertebrates. Protoplasma 252:489–503
Gorelova O, Baulina O, Solovchenko A, Selyakh I, Chivkunova O, Semenova L, Scherbakov P, Burakova O, Lobakova E (2015b) Coordinated rearrangements of assimilatory and storage cell compartments in a nitrogen-starving symbiotic chlorophyte cultivated under high light. Arch Microbiol 197:181–195
Horton P (2014) Developments in research on non-photochemical fluorescence quenching: emergence of key ideas, theories and experimental approaches. In: Demmig-Adams B, Garab G, Adams W III, Govindjee (eds) Non-photochemical quenching and energy dissipation in plants, algae and cyanobacteria. Springer, Netherlands, pp 73–95
Huner N, Dahal K, Hollis L, Bode R, Rosso D, Krol M, Ivanov AG (2012) Chloroplast redox imbalance governs phenotypic plasticity: the “grand design of photosynthesis” revisited. Front Plant Sci 3. https://doi.org/10.3389/fpls.2012.00255
Johnson M, Brain A, Ruban A (2011) Changes in thylakoid membrane thickness associated with the reorganization of photosystem II light harvesting complexes during photoprotective energy dissipation. Plant Signal Behav 6:1386–1390
Jungnick N, Ma Y, Mukherjee B, Cronan JC, Speed DJ, Laborde SM, Longstreth DJ, Moroney JV (2014) The carbon concentrating mechanism in Chlamydomonas reinhardtii: finding the missing pieces. Photosynth Res 121:159–173
Maxwell K, Johnson G (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Minamikawa T, Toyooka K, Okamoto T, Hara-Nishimura I, Nishimura M (2001) Degradation of ribulose-bisphosphate carboxylase by vacuolar enzymes of senescing French bean leaves: immunocytochemical and ultrastructural observations. Protoplasma 218:144–153
Miyachi S, Tsuzuki M, Maruyama I, Gantar M, Miyachi S, Matsushima H (1986) Effects of CO2 concentration during growth on the intracellular structure of Chlorella and Scenedesmus (Chlorophyta). J Phycol 22:313–319
Miyachi S, Iwasaki I, Shiraiwa Y (2003) Historical perspective on microalgal and cyanobacterial acclimation to low- and extremely high-CO2 conditions. Photosynth Res 77:139–153
Muradyan EA, Klyachko-Gurvich GL, Tsoglin LN, Sergeyenko TV, Pronina NA (2004) Changes in lipid metabolism during adaptation of the Dunaliella salina photosynthetic apparatus to high CO2 concentration. Rus J Plant Physiol 51:53–62
Ptushenko V, Solovchenko A (2016) Tolerance of the photosynthetic apparatus to acidification of the growth medium as a possible determinant of CO2-tolerance of the symbiotic microalga Desmodesmus sp. IPPAS-2014. Biochem Mosc 81:1531–1537
Raeesossadati MJ, Ahmadzadeh H, McHenry MP, Moheimani NR (2014) CO2 bioremediation by microalgae in photobioreactors: impacts of biomass and CO2 concentrations, light, and temperature. Algal Res 6:78–85
Reynolds E (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Ruban AV (2016) Non-photochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protection against photodamage. Plant Physiol 170:1903–1916
Seckbach J, Baker FA, Shugarman PM (1970) Algae thrive under pure CO2. Nature 227:744–745
Shebanova A, Ismagulova T, Solovchenko A, Baulina O, Lobakova E, Ivanova A, Moiseenko A, Shaitan K, Polshakov V, Nedbal L, Gorelova O (2017) Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 254:1323–1340
Solovchenko A, Khozin-Goldberg I (2013) High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol Lett 35:1745–1752
Solovchenko A, Chivkunova O, Semenova L, Selyakh I, Shcherbakov P, Karpova E, Lobakova E (2013a) Stress-induced changes in pigment and fatty acid content in the microalga Desmodesmus sp. isolated from a White Sea hydroid. Russ J Plant Physiol 60:313–321
Solovchenko A, Solovchenko O, Khozin-Goldberg I, Didi-Cohen S, Pal D, Cohen Z, Boussiba S (2013b) Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Res 2:175–182
Solovchenko A, Gorelova O, Selyakh I, Semenova L, Chivkunova O, Baulina O, Lobakova E (2014) Desmodesmus sp. 3Dp86E-1-a novel symbiotic chlorophyte capable of growth on pure CO2. Mar Biotechnol 16:495–501
Solovchenko A, Gorelova O, Selyakh I, Pogosyan S, Baulina O, Semenova L, Chivkunova O, Voronova E, Konyukhov I, Shcherbakov P, Lobakova E (2015) A novel CO2-tolerant symbiotic Desmodesmus (Chlorophyceae, Desmodesmaceae): acclimation to and performance at a high carbon dioxide level. Algal Res 11:399–410
Solovchenko A, Gorelova O, Selyakh I, Baulina O, Semenova L, Logacheva M, Chivkunova O, Shcherbakov P, Lobakova E (2016) Nitrogen availability modulates CO2 tolerance in a symbiotic chlorophyte. Algal Res 16:177–188
Tikhonov A (2015) Induction events and short-term regulation of electron transport in chloroplasts. Photosynth Res 125:65–94
Van Den Hende S, Vervaeren H, Boon N (2012) Flue gas compounds and microalgae: (bio-) chemical interactions leading to biotechnological opportunities. Biotechnol Adv 30:1405–1424
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2014) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Wittenbach VA, Lin W, Hebert RR (1982) Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol 69:98–102
Yamano T, Tsujikawa T, Hatano K, Ozawa S-i, Takahashi Y, Fukuzawa H (2010) Light and low-CO2-dependent LCIB–LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51:1453–1468
Acknowledgements
Molecular identification of Tetradesmus obliquus IPPAS S-2023 was funded by the Russian Science Foundation (grant 14-50-00029), and the CO2 tolerance investigation was supported by Russian Foundation for Basic research via the grants 15-54-06004 (ultrastructural studies) and 15-04-01061 (physiological studies). The ultrastructure studies were carried out at the User Facilities Center of M.V. Lomonosov Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
(DOC 468 kb)
Rights and permissions
About this article
Cite this article
Scherbakov, P., Ismagulova, T., Chernov, T. et al. A new subarctic strain of Tetradesmus obliquus. Part II: comparative studies of CO2-stress tolerance. J Appl Phycol 30, 2751–2761 (2018). https://doi.org/10.1007/s10811-017-1334-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1334-9