Abstract
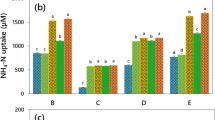
Mutual interactions in co-cultures of microalgae and bacteria are well known for establishing consortia and nutrient uptake in aquatic habitats, but the phenotypic changes in terms of morphological, physiological, and biochemical attributes that drive these interactions have not been clearly understood. In this novel study, we demonstrated the phenotypic response in a co-culture involving a microalga, Tetradesmus obliquus IS2, and a bacterium, Variovorax paradoxus IS1, grown with varying concentrations of two inorganic nitrogen sources. Modified Bold’s basal medium was supplemented with five ratios (%) of NO3-N:NH4-N (100:0, 75:25, 50:50, 25:75, and 0:100), and by maintaining N:P Redfield ratio of 16:1. The observed morphological changes in microalga included an increase in granularity and a broad range of cell sizes under the influence of increased ammonium levels. Co-culturing in presence of NO3-N alone or combination with NH4-N up to equimolar concentrations resulted in complete nitrogen uptake, increased growth in both the microbial strains, and enhanced accumulation of carbohydrates, proteins, and lipids. Total chlorophyll content in microalga was also significantly higher when it was grown as a co-culture with NO3-N and NH4-N up to a ratio of 50:50. Significant upregulation in the synthesis of amino acids and sugars and downregulation of organic acids were evident with higher ammonium uptake in the co-culture, indicating the regulation of carbon and nitrogen assimilation pathways and energy synthesis. Our data suggest that the co-culture of strains IS1 and IS2 could be exploited for effluent treatment by considering the concentrations of inorganic sources, particularly ammonium, in the wastewaters.




Similar content being viewed by others

References
Perera I, Subashchandrabose SR, Venkateswarlu K, Naidu R, Megharaj M (2018) Consortia of cyanobacteria/microalgae and bacteria in desert soils: an underexplored microbiota. Appl Microbiol Biotechnol 102:7351–7363. https://doi.org/10.1007/s00253-018-9192-1
Perera IA, Abinandan S, Subashchandrabose SR, Venkateswarlu K, Naidu R, Megharaj M (2019) Advances in the technologies for studying consortia of bacteria and cyanobacteria/microalgae in wastewaters. Crit Rev Biotechnol 39:709–731. https://doi.org/10.1080/07388551.2019.1597828
Perera IA, Abinandan S, Subashchandrabose SR, Venkateswarlu K, Naidu R, Megharaj M (2021) Microalgal–bacterial consortia unveil distinct physiological changes to facilitate growth of microalgae. FEMS Microbiol Ecol 97:fiab012. https://doi.org/10.1093/femsec/fiab012
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29:896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009
Higgins BT, Gennity I, Fitzgerald PS, Ceballos SJ, Fiehn O, VanderGheynst JS (2018) Algal–bacterial synergy in treatment of winery wastewater. npj Clean Water 1:6. https://doi.org/10.1038/s41545-018-0005-y
Peng H, de-Bashan LE, Bashan Y, Higgins BT, (2020) Indole-3-acetic acid from Azosprillum brasilense promotes growth in green algae at the expense of energy storage products. Algal Res 47:101845. https://doi.org/10.1016/j.algal.2020.101845
Su Y, Mennerich A, Urban B (2012) Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: influence of algae and sludge inoculation ratios. Bioresour Technol 105:67–73. https://doi.org/10.1016/j.biortech.2011.11.113
Higgins BT, Labavitch JM, VanderGheynst JS (2015) Co-culturing Chlorella minutissima with Escherichia coli can increase neutral lipid production and improve biodiesel quality. Biotechnol Bioeng 112:1801–1809. https://doi.org/10.1002/bit.25609
Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M (2018) Microalgae–bacteria biofilms: a sustainable synergistic approach in remediation of acid mine drainage. Appl Microbiol Biotechnol 102:1131–1144. https://doi.org/10.1007/s00253-017-8693-7
Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M (2019) Soil microalgae and cyanobacteria: the biotechnological potential in the maintenance of soil fertility and health. Crit Rev Biotechnol 39:981–998. https://doi.org/10.1080/07388551.2019.1654972
Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176. https://doi.org/10.1146/annurev.micro.57.030502.090820
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y, (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36. https://doi.org/10.1016/j.watres.2010.08.037
Gunka K, Commichau FM (2012) Control of glutamate homeostasis in Bacillus subtilis: a complex interplay between ammonium assimilation, glutamate biosynthesis and degradation. Mol Microbiol 85:213–224. https://doi.org/10.1111/j.1365-2958.2012.08105.x
Glibert PM, Wilkerson FP, Dugdale RC, Raven JA, Dupont CL, Leavitt PR, Parker AE, Burkholder JM, Kana TM (2016) Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol Oceanogr 61:165–197. https://doi.org/10.1002/lno.10203
Subramaniyam V, Subashchandrabose SR, Ganeshkumar V, Thavamani P, Chen Z, Naidu R, Megharaj M (2016) Cultivation of Chlorella on brewery wastewater and nano-particle biosynthesis by its biomass. Bioresour Technol 211:698–703. https://doi.org/10.1016/j.biortech.2016.03.154
Ganeshkumar V, Subashchandrabose SR, Dharmarajan R, Venkateswarlu K, Naidu R, Megharaj M (2018) Use of mixed wastewaters from piggery and winery for nutrient removal and lipid production by Chlorella sp. MM3. Bioresour Technol 256:254–258. https://doi.org/10.1016/j.biortech.2018.02.025
Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M (2018) Nutrient removal and biomass production: advances in microalgal biotechnology for wastewater treatment. Crit Rev Biotechnol 38:1244–1260. https://doi.org/10.1080/07388551.2018.1472066
Malerba ME, Heimann K, Connolly SR (2016) Nutrient utilization traits vary systematically with intraspecific cell size plasticity. Funct Ecol 30:1745–1755. https://doi.org/10.1111/1365-2435.12662
Liu J, Xia D, Qiu W (2021) Exploiting microalgal competition ability to acquire nitrogen and light. Phycol Res 69:66–76. https://doi.org/10.1111/pre.12441
Lachmann SC, Mettler-Altmann T, Wacker A, Spijkerman E (2019) Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol Evol 9:1070–1082. https://doi.org/10.1002/ece3.4790
Chubukov V, Gerosa L, Kochanowski K, Sauer U (2014) Coordination of microbial metabolism. Nat Rev Microbiol 12:327–340. https://doi.org/10.1038/nrmicro3238
Tibocha-Bonilla JD, Kumar M, Richelle A, Godoy-Silva RD, Zengler K, Zuñiga C (2020) Dynamic resource allocation drives growth under nitrogen starvation in eukaryotes. npj Syst Biol Appl 6:14. https://doi.org/10.1038/s41540-020-0135-y
Mandal S, Shurin JB, Efroymson RA, Mathews TJ (2018) Heterogeneity in nitrogen sources enhances productivity and nutrient use efficiency in algal polycultures. Environ Sci Technol 52:3769–3776. https://doi.org/10.1021/acs.est.7b05318
Perera IA, Abinandan S, Subashchandrabose SR Venkateswarlu K, Cole N, Naidu R, Megharaj M (2021) Extracellular polymeric substances drive symbiotic interactions in bacterial‒microalgal consortia. Microbial Ecol. https://doi.org/10.1007/s00248-021-01772-1
Levitan O, Dinamarca J, Zelzion E, Lun DS, Guerra LT, Kim MK, Kim J, Van Mooy BAS, Bhattacharya D, Falkowski PG (2015) Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutumunder nitrogen stress. Proc Natl Acad Sci 112:412–417. https://doi.org/10.1073/pnas.1419818112
Klausmeier CA, Litchman E, Daufresne T, Levin SA (2004) Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429:171–174. https://doi.org/10.1038/nature02454
Abinandan S, Subashchandrabose SR, Cole N, Dharmarajan R, Venkateswarlu K, Megharaj M (2019) Sustainable production of biomass and biodiesel by acclimation of non-acidophilic microalgae to acidic conditions. Bioresour Technol 271:316–324. https://doi.org/10.1016/j.biortech.2018.09.140
Chen Y, Vaidyanathan S (2013) Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal Chim Acta 776:31–40. https://doi.org/10.1016/j.aca.2013.03.005
Kim HK, Choi YH, Verpoorte R (2010) NMR-based metabolomic analysis of plants. Nat Protoc 5:536–549. https://doi.org/10.1038/nprot.2009.237
Arora N, Dubey D, Sharma M, Patel A, Guleria A, Pruthi PA, Kumar D, Pruthi V, Poluri KM (2018) NMR-based metabolomic approach to elucidate the differential cellular responses during mitigation of arsenic (III, V) in a green microalga. ACS Omega 3:11847–11856. https://doi.org/10.1021/acsomega.8b01692
Abinandan S, Perera IA, Subashchandrabose SR, Venkateswarlu K, Cole N, Megharaj M (2020) Acid-adapted microalgae exhibit phenotypic changes for their survival in acid mine drainage samples. FEMS Microbiol Ecol 96:fiaa113. https://doi.org/10.1093/femsec/fiaa113
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494. https://doi.org/10.1093/nar/gky310
Redfield AC (1958) The biological control of chemical factors in the environment. Amer Sci 46:205–221. http://www.jstor.org/stable/27827150
Hillebrand H, Sommer U (1999) The nutrient stoichiometry of benthic microalgal growth: Redfield proportions are optimal. Limnol Oceanogr 44:440–446. https://doi.org/10.4319/lo.1999.44.2.0440
Liu J, Li Z, Guo J-s, Xiao Y, Fang F, Qin R-c, Zhang L-l (2017) The effect of light on the cellular stoichiometry of Chlorella sp. in different growth phases: implications of nutrient drawdown in batch experiments. J Appl Phycol 29:123–131. https://doi.org/10.1007/s10811-016-0962-9
Jiang R, Qin L, Feng S, Huang D, Wang Z, Zhu S (2021) The joint effect of ammonium and pH on the growth of Chlorella vulgaris and ammonium removal in artificial liquid digestate. Bioresour Technol 325:124690. https://doi.org/10.1016/j.biortech.2021.124690
Finkel ZV, Beardall J, Flynn KJ, Quigg A, Rees TAV, Raven JA (2009) Phytoplankton in a changing world: cell size and elemental stoichiometry. J Plankton Res 32:119–137. https://doi.org/10.1093/plankt/fbp098
Flynn KJ, Skibinski DOF, Lindemann C (2018) Effects of growth rate, cell size, motion, and elemental stoichiometry on nutrient transport kinetics. PLoS Comput Biol 14:e1006118. https://doi.org/10.1371/journal.pcbi.1006118
Flynn KJ, Fasham MJR, Hipkin CR (1997) Modelling the interactions between ammonium and nitrate uptake in marine phytoplankton. Phil Trans R Soc Lond B 352:1625–1645. https://doi.org/10.1098/rstb.1997.0145
Smith SR, Dupont CL, McCarthy JK, Broddrick JT, Oborník M, Horák A, Füssy Z, Cihlář J, Kleessen S, Zheng H, McCrow JP, Hixson KK, Araújo WL, Nunes-Nesi A, Fernie A, Nikoloski Z, Palsson BO, Allen AE (2019) Evolution and regulation of nitrogen flux through compartmentalized metabolic networks in a marine diatom. Nat Commun 10:4552. https://doi.org/10.1038/s41467-019-12407-y
Hachiya T, Sakakibara H (2016) Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J Exp Bot 68:2501–2512. https://doi.org/10.1093/jxb/erw449
Fernandez E, Galvan A (2007) Inorganic nitrogen assimilation in Chlamydomonas. J Exp Bot 58:2279–2287. https://doi.org/10.1093/jxb/erm106
Li T, Chen X, Lin S (2021) Physiological and transcriptomic responses to N-deficiency and ammonium: nitrate shift in Fugacium kawagutii (Symbiodiniaceae). Sci Total Environ 753:141906. https://doi.org/10.1016/j.scitotenv.2020.141906
Le Chevanton M, Garnier M, Bougaran G, Schreiber N, Lukomska E, Bérard JB, Fouilland E, Bernard O, Cadoret JP (2013) Screening and selection of growth-promoting bacteria for Dunaliella cultures. Algal Res 2:212–222. https://doi.org/10.1016/j.algal.2013.05.003
Schmollinger S, Mühlhaus T, Boyle NR, Blaby IK, Casero D, Mettler T, Moseley JL, Kropat J, Sommer F, Strenkert D, Hemme D, Pellegrini M, Grossman AR, Stitt M, Schroda M, Merchant SS (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26:1410–1435. https://doi.org/10.1105/tpc.113.122523
Martin GJO, Hill DRA, Olmstead ILD, Bergamin A, Shears MJ, Dias DA, Kentish SE, Scales PJ, Botté CY, Callahan DL (2014) Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae). PLoS One 9:e103389. https://doi.org/10.1371/journal.pone.0103389
Cheng J-S, Niu Y-H, Lu S-H, Yuan Y-J (2012) Metabolome analysis reveals ethanolamine as potential marker for improving lipid accumulation of model photosynthetic organisms. J Chem Technol Biotechnol 87:1409–1418. https://doi.org/10.1002/jctb.3759
Wang J, Zhou W, Chen H, Zhan J, He C, Wang Q (2019) Ammonium nitrogen tolerant chlorella strain screening and its damaging effects on photosynthesis. Front Microbiol 9:3250. https://doi.org/10.3389/fmicb.2018.03250
Acknowledgements
IAP acknowledges the Australian Government RTP scholarship, and SRS acknowledges the University of Newcastle for ECR HDR scholarship and CRC CARE for support. We thank Nicole Cole, Analytical and Biomolecular Research Facility (ABRF), the University of Newcastle, for help with flow cytometry analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perera, I.A., Abinandan, S., Subashchandrabose, S.R. et al. Impact of Nitrate and Ammonium Concentrations on Co-Culturing of Tetradesmus obliquus IS2 with Variovorax paradoxus IS1 as Revealed by Phenotypic Responses. Microb Ecol 83, 951–959 (2022). https://doi.org/10.1007/s00248-021-01832-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01832-6