Abstract
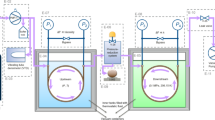
Accurate thermophysical properties will be essential to select and evaluate cryogenic fluids. The viscosity data of common cryogenic fluids was scattered below 200 K. To verify the data and reference correlations, in this work, the viscosity of nitrogen and argon in gas, liquid, and supercritical states were measured by using a vibrating-wire viscometer at temperatures between 90 K and 200 K and pressures between 0.3 MPa and 5 MPa. The standard uncertainty of the viscosity measurements was estimated to be 2.1 % over all temperature and pressure ranges. The results together with literature data were compared with the reference correlations. The results show good agreement between our measurements and those of the reference correlations within 2 %.






Similar content being viewed by others
References
M. Mia, M.A. Khan, N.R. Dhar, Int. J. Adv. Manuf. Technol. 93, 975 (2017). https://doi.org/10.1007/s00170-017-0566-9
I.A. Burkov, A.V. Pushkarev, S.S. Ryabikin, A.V. Shakurov, D.I. Tsiganov, A.A. Zherdev, Int. J. Refrig. 133, 30 (2022). https://doi.org/10.1016/j.ijrefrig.2021.10.020
W.J. Bailey, H. Arif, Cryogenics 32, 221 (1992). https://doi.org/10.1016/0011-2275(92)90271-B
M.J. Tuinier, M. van Sint Annaland, G.J. Kramer, J.A.M. Kuipers, Chem. Eng. Sci. 65, 114 (2010). https://doi.org/10.1016/j.ces.2009.01.055
C. Hilbert, U. Ghoshal, H. Kroger, J.S. Martens, V.M. Hietala, T.A. Plut, Appl. Phys. Lett. 64, 2442 (1994). https://doi.org/10.1063/1.111593
D. Babikova, A. Petrov, I.O.P. Conf, Ser. Mater. Sci. Eng. 779, 012010 (2020). https://doi.org/10.1088/1757-899X/779/1/012010
R. Xue, L. Chen, X. Zhong, X. Liu, S. Chen, Y. Hou, Cryogenics 97, 144 (2019). https://doi.org/10.1016/j.cryogenics.2018.09.010
A.A. Sam, P. Ghosh, Cryogenics 82, 1 (2017). https://doi.org/10.1016/j.cryogenics.2017.01.004
R.L. Oonk, D.C. Hustvedt, in Advances in Cryogenic Engineering, vol. 31, ed. by R.W. Fast (Springer, Boston, 1986), pp.415–422
C.E. Ejim, M.A. Rahman, A. Amirfazli, B.A. Fleck, Fuel 89, 1872 (2010). https://doi.org/10.1016/j.fuel.2010.03.005
D.E. Diller, Phys. A Stat. Mech. Appl. 119, 92 (1983). https://doi.org/10.1016/0378-4371(83)90149-8
S. Förster, Cryogenics 3, 176 (1963). https://doi.org/10.1016/0011-2275(63)90012-2
W. Grevendonk, W. Herreman, A. De Bock, Physica 46, 600 (1970). https://doi.org/10.1016/0031-8914(70)90148-5
J. Hellemans, H. Zink, O. Van Paemel, Physica 47, 45 (1970). https://doi.org/10.1016/0031-8914(70)90098-4
W.M. Haynes, Physica 67(3), 440 (1973). https://doi.org/10.1016/0031-8914(73)90162-6
B.Y. Baharudin, D.A. Jackson, P.E. Schoen, J. Rouch, Phys. Lett. A 51, 409 (1975). https://doi.org/10.1016/0375-9601(75)90750-1
A. De Bock, W. Grevendonk, W. Herreman, Physica 37, 227 (1967). https://doi.org/10.1016/0031-8914(67)90153-X
J. Hellemans, H. Zink, O. Van Paemel, Physica 46, 395 (1970). https://doi.org/10.1016/0031-8914(70)90013-3
E.W. Lemmon, R.T. Jacobsen, Int. J. Thermophys. 25, 21 (2004). https://doi.org/10.1023/B:IJOT.0000022327.04529.f3
J.T. Tough, W.D. McCormick, J.G. Dash, Rev. Sci. Instrum. 35, 1345 (2004). https://doi.org/10.1063/1.1718741
W. Ruesink, J.P. Harrison, A. Sachrajda, J. Low Temp. Phys. 70, 393 (1988). https://doi.org/10.1007/BF00682788
D.C. Carless, H.E. Hall, J.R. Hook, J. Low Temp. Phys. 50, 583 (1983). https://doi.org/10.1007/BF00683497
T. Retsina, S.M. Richardson, W.A. Wakeham, Appl. Sci. Res. 43, 127 (1986). https://doi.org/10.1007/BF00386040
T. Retsina, S.M. Richardson, W.A. Wakeham, Appl. Sci. Res. 43, 325 (1987). https://doi.org/10.1007/BF00540567
A.A.H. Pádua, J.M.N.A. Fareleira, J.C.G. Calado, W.A. Wakeham, Int. J. Thermophys. 17, 781 (1996). https://doi.org/10.1007/BF01439190
M.E. Kandil, K.N. Marsh, A.R.H. Goodwin, J. Chem. Eng. Data 50, 647 (2005). https://doi.org/10.1021/je049636m
J. Wilhelm, E. Vogel, J.K. Lehmann, W.A. Wakeham, Int. J. Thermophys. 19(2), 391 (1998). https://doi.org/10.1023/A:1022505209226
X. Meng, J. Zhang, J. Wu, J. Chem. Eng. Data 56, 4956 (2011). https://doi.org/10.1021/je200854k
R. Span, E.W. Lemmon, R.T. Jacobsen, W. Wagner, A. Yokozeki, J. Phys. Chem. Ref. Data 29, 1361 (2000). https://doi.org/10.1063/1.1349047
M.E. Kandil, (University of Canterbury, Christchurch, 2005), p. 18
P.R. Bevington, D.K. Robinson, Data Reduction and Error Analysis for the Physical Sciences. (McGraw-Hill, Boston, 2003), p. 104–106
C. Tegeler, R. Span, W. Wagner, J. Phys. Chem. Ref. Data 28, 779 (1999). https://doi.org/10.1063/1.556037
E. Lassner, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, 1st edn. (Kluwer Academic/Plenum Publishers, New York, 1999), p.324
D.W. Gough, G.P. Matthews, E.B. Smith, J. Chem. Soc. Faraday Trans. 1 72, 645 (1976). https://doi.org/10.1039/F19767200645
J.T.F. Kao, R. Kobayashi, J. Chem. Phys. 47, 2836 (1967). https://doi.org/10.1063/1.1712306
H.L. Johnston, K.E. McCloskey, J. Phys. Chem. 44, 1038 (1940). https://doi.org/10.1021/j150405a004
J.A. Gracki, G.P. Flynn, J. Ross, J. Chem. Phys. 44, 3856 (1969). https://doi.org/10.1063/1.1672602
A.G. Clarke, E.B. Smith, J. Chem. Phys. 48, 3988 (1968). https://doi.org/10.1063/1.1669725
H.L. Johnston, E.R. Grilly, J. Phys. Chem. 46, 948 (1942). https://doi.org/10.1021/j150422a019
W.M. Haynes, D.E. Diller, H.M. Roder, Cryogenics 27, 348 (1987). https://doi.org/10.1016/0011-2275(87)90206-2
Funding
The authors acknowledge the financial support of the National Natural Science Foundation of China (No. 51976164).
Author information
Authors and Affiliations
Contributions
XZ performed the measurements, prepared the figures and tables, and wrote the main manuscript text. WQ contributed to experimental measurements and made modifications to the manuscript. ZL contributed to experimental measurements and carried out the evaluation of the literature data. JW and XM proposed the research content and made modifications to the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Qin, W., Li, Z. et al. Viscosity Measurements of Nitrogen and Argon at Temperatures from 90 K to 200 K and Pressures Up To 5 MPa. Int J Thermophys 45, 30 (2024). https://doi.org/10.1007/s10765-023-03325-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03325-9