Abstract
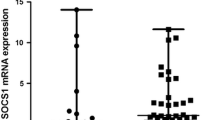
Increasing evidence has demonstrated the association between UBASH3A gene and multiple autoimmune diseases (ADs). The aim of our study was to explore the potential effect of UBASH3A messenger RNA (mRNA) expression and its role in the pathogenesis of systemic lupus erythematosus (SLE). UBASH3A mRNA levels were detected by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) in total RNA, isolated from the peripheral blood mononuclear cells (PBMCs) of 32 SLE patients and 30 healthy donors with TRIzol Reagent. The expression level of UBASH3A mRNA was significantly reduced in PBMCs from SLE patients when compared with healthy controls (p = 0.002). UBASH3A mRNA expression levels in lower active SLE were significantly lower than that in inactive SLE groups (p = 0.000). There was a negative association between mRNA levels of hyper-active and lower-active SLE patients (p = 0.000). Moreover, a significant negative correlation between UBASH3A mRNA expression and the onset age of SLE patients was found (p = 0.044). A negative correlation was found between UBASH3A mRNA expression and SLEDAI (p = 0.049). Nevertheless, no significant difference was found between patients with lupus nephritis (LN) and those without LN (p = 0.392). The presence of leukopenia, positive for anti-dsDNA antibody and anti-SSB antibody were associated with UBASH3A mRNA levels in SLE patients (all p < 0.05). The dysregulation of UBASH3A mRNA levels in SLE patients and their correlations with experimental parameters suggested that UBASH3A may involve in the pathogenesis of SLE.


Similar content being viewed by others
References
Zhou, M., L.H. Li, H. Peng, R. Li, C.C. Feng, W.D. Xu, R.X. Leng, H.F. Pan, and D.Q. Ye. 2014. Decreased ITGAM and FcgammaRIIIA mRNA expression levels in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clinical and Experimental Medicine 14: 269–274.
Tang, Z.M., M. Fang, J.P. Wang, P.C. Cai, P. Wang, and L.H. Hu. 2014. Clinical relevance of plasma miR-21 in new-onset systemic lupus erythematosus patients. Journal of Clinical Laboratory Analysis 28: 446–451.
Palafox-Sanchez, C.A., E. Oregon-Romero, D.C. Salazar-Camarena, Y.M. Valle, J.R. Machado-Contreras, A. Cruz, M. Orozco-Lopez, G.. Orozco-Barocio, M. Vazquez-Del Mercado, and J.F. Muñoz-Valle. 2014. Munoz-Valle. Association of interleukin-10 promoter haplotypes with disease susceptibility and IL-10 levels in Mexican patients with systemic lupus erythematosus. Clin Exp Med. doi:10.1007/s10238-014-0315-4.
Hom, G., R.R. Graham, B. Modrek, K.E. Taylor, W. Ortmann, S. Garnier, A.T. Lee, S.A. Chung, R.C. Ferreira, P.V. Pant, D.G. Ballinger, R. Kosoy, F.Y. Demirci, M.I. Kamboh, A.H. Kao, C. Tian, I. Gunnarsson, A.A. Bengtsson, S. Rantapää-Dahlqvist, M. Petri, S. Manzi, M.F. Seldin, L. Rönnblom, A.C. Syvänen, L.A. Criswell, P.K. Gregersen, and T.W. Behrens. 2008. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. New England Journal of Medicine 358: 900–909.
Kozyrev, S.V., A.K. Abelson, J. Wojcik, A. Zaghlool, M.V. Linga Reddy, E. Sanchez, I. Gunnarsson, E. Svenungsson, G. Sturfelt, A. Jonsen, L. Truedsson, B.A. Pons-Estel, T. Witte, S. D’Alfonso, N. Barizzone, M.G. Danieli, C. Gutierrez, A. Suarez, P. Junker, H. Laustrup, M.F. González-Escribano, J. Martin, H. Abderrahim, and M.E. Alarcón-Riquelme. 2008. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nature Genetics 40: 211–216.
Harley, J.B., M.E. Alarcon-Riquelme, L.A. Criswell, C.O. Jacob, R.P. Kimberly, K.L. Moser, B.P. Tsao, T.J. Vyse, C.D. Langefeld, S.K. Nath, J.M. Guthridge, B.L. Cobb, D.B. Mirel, M.C. Marion, A.H. Williams, J. Divers, W. Wang, S.G. Frank, B. Namjou, S.B. Gabriel, A.T. Lee, P.K. Gregersen, T.W. Behrens, K.E. Taylor, M. Fernando, R. Zidovetzki, P.M. Gaffney, J.C. Edberg, J.D. Rioux, J.O. Ojwang, J.A. James, J.T. Merrill, G.S. Gilkeson, M.F. Seldin, H. Yin, E.C. Baechler, Q.Z. Li, E.K. Wakeland, G.R. Bruner, K.M. Kaufman, and J.A. Kelly. 2008. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature Genetics 40: 204–210.
Han, J.W., H.F. Zheng, Y. Cui, L.D. Sun, D.Q. Ye, Z. Hu, J.H. Xu, Z.M. Cai, W. Huang, G.P. Zhao, H.F. Xie, H. Fang, Q.J. Lu, J.H. Xu, X.P. Li, Y.F. Pan, D.Q. Deng, F.Q. Zeng, Z.Z. Ye, X.Y. Zhang, Q.W. Wang, F. Hao, L. Ma, X.B. Zuo, F.S. Zhou, W.H. Du, Y.L. Cheng, J.Q. Yang, S.K. Shen, J. Li, Y.J. Sheng, X.X. Zuo, W.F. Zhu, F. Gao, P.L. Zhang, Q. Guo, B. Li, M. Gao, F.L. Xiao, C. Quan, C. Zhang, Z. Zhang, K.J. Zhu, Y. Li, D.Y. Hu, W.S. Lu, J.L. Huang, S.X. Liu, H. Li, Y.Q. Ren, Z.X. Wang, C.J. Yang, P.G. Wang, W.M. Zhou, Y.M. Lv, A.P. Zhang, S.Q. Zhang, D. Lin, Y. Li, H.Q. Low, M. Shen, Z.F. Zhai, Y. Wang, F.Y. Zhang, S. Yang, J.J. Liu, and X.J. Zhang. 2009. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nature Genetics 41: 1234–1237.
Graham, R.R., C. Cotsapas, L. Davies, R. Hackett, C.J. Lessard, J.M. Leon, N.P. Burtt, C. Guiducci, M. Parkin, C. Gates, R.M. Plenge, T.W. Behrens, J.E. Wither, J.D. Rioux, P.R. Fortin, D.C. Graham, A.K. Wong, T.J. Vyse, M.J. Daly, D. Altshuler, K.L. Moser, and P.M. Gaffney. 2008. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nature Genetics 40: 1059–1061.
Cervino, A.C., N.F. Tsinoremas, and R.W. Hoffman. 2007. A genome-wide study of lupus: preliminary analysis and data release. Annals of the New York Academy of Sciences 1110: 131–139.
Stanford, S.M., V. Ahmed, A.M. Barrios, and N. Bottini. 2014. Cellular biochemistry methods for investigating protein tyrosine phosphatases. Antioxidants & Redox Signaling 20: 2160–2178.
Rigden, D.J. 2008. The histidine phosphatase superfamily: structure and function. The Biochemical Journal 409: 333–348.
Wattenhofer, M., K. Shibuya, J. Kudoh, R. Lyle, J. Michaud, C. Rossier, K. Kawasaki, S. Asakawa, S. Minoshima, A. Berry, B. Bonne-Tamir, N. Shimizu, S.E. Antonarakis, and H.S. Scott. 2001. Isolation and characterization of the UBASH3A gene on 21q22.3 encoding a potential nuclear protein with a novel combination of domains. Human Genetics 108: 140–147.
Tsygankov, A.Y. 2008. Multidomain STS/TULA proteins are novel cellular regulators. IUBMB Life 60: 224–231.
Diaz-Gallo, L.M., E. Sanchez, N. Ortego-Centeno, J.M. Sabio, F.J. Garcia-Hernandez, E. de Ramon, M.A. Gonzalez-Gay, W. Torsten, H.J. Anders, M.F. Gonzalez-Escribano, and J. Martin. 2013. Evidence of new risk genetic factor to systemic lupus erythematosus: the UBASH3A gene. PloS One 8, e60646.
Frederiksen, B.N., A.K. Steck, M. Kroehl, M.M. Lamb, R. Wong, M. Rewers, and J.M. Norris. 2013. Evidence of stage- and age-related heterogeneity of non-HLA SNPs and risk of islet autoimmunity and type 1 diabetes: the diabetes autoimmunity study in the young. Clinical and Developmental Immunology 2013: 417657.
Kim, K., S.Y. Bang, H.S. Lee, S.K. Cho, C.B. Choi, Y.K. Sung, T.H. Kim, J.B. Jun, D.H. Yoo, Y.M. Kang, S.K. Kim, C.H. Suh, S.C. Shim, S.S. Lee, J. Lee, W.T. Chung, J.Y. Choe, H.D. Shin, J.Y. Lee, B.G. Han, S.K. Nath, S. Eyre, J. Bowes, D.A. Pappas, J.M. Kremer, M.A. Gonzalez-Gay, L. Rodriguez-Rodriguez, L. Ärlestig, Y. Okada, D. Diogo, K.P. Liao, E.W. Karlson, S. Raychaudhuri, S. Rantapää-Dahlqvist, J. Martin, L. Klareskog, L. Padyukov, P.K. Gregersen, J. Worthington, J.D. Greenberg, R.M. Plenge, and S.C. Bae. 2015. High-density genotyping of immune loci in Koreans and Europeans identifies eight new rheumatoid arthritis risk loci. Annals of the Rheumatic Diseases 74, e13.
Plagnol, V., J.M. Howson, D.J. Smyth, N. Walker, J.P. Hafler, C. Wallace, H. Stevens, L. Jackson, M.J. Simmonds, P.J. Bingley, S.C. Gough, and J.A. Todd. 2011. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genetics 7, e1002216.
Grant, S.F., H.Q. Qu, J.P. Bradfield, L. Marchand, C.E. Kim, J.T. Glessner, R. Grabs, S.P. Taback, E.C. Frackelton, A.W. Eckert, K. Annaiah, M.L. Lawson, F.G. Otieno, E. Santa, J.L. Shaner, R.M. Smith, R. Skraban, M. Imielinski, R.M. Chiavacci, R.W. Grundmeier, C.A. Stanley, S.E. Kirsch, D. Waggott, A.D. Paterson, D.S. Monos, DCCT/EDIC Research Group, C. Polychronakos, and H. Hakonarson. 2009. Follow-up analysis of genome-wide association data identifies novel loci for type 1 diabetes. Diabetes 58: 290–295.
Jin, Y., S.A. Birlea, P.R. Fain, K. Gowan, S.L. Riccardi, P.J. Holland, C.M. Mailloux, A.J. Sufit, S.M. Hutton, A. Amadi-Myers, D.C. Bennett, M.R. Wallace, W.T. McCormack, E.H. Kemp, D.J. Gawkrodger, A.P. Weetman, M. Picardo, G. Leone, A. Taïeb, T. Jouary, K. Ezzedine, N. van Geel, J. Lambert, A. Overbeck, and R.A. Spritz. 2010. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. New England Journal of Medicine 362: 1686–1697.
Zhernakova, A., E.A. Stahl, G. Trynka, S. Raychaudhuri, E.A. Festen, L. Franke, H.J. Westra, R.S. Fehrmann, F.A. Kurreeman, B. Thomson, N. Gupta, J. Romanos, R. McManus, A.W. Ryan, G. Turner, E. Brouwer, M.D. Posthumus, E.F. Remmers, F. Tucci, R. Toes, E. Grandone, M.C. Mazzilli, A. Rybak, B. Cukrowska, M.J. Coenen, T.R. Radstake, P.L. van Riel, Y. Li, P.I. de Bakker, P.K. Gregersen, J. Worthington, K.A. Siminovitch, L. Klareskog, T.W. Huizinga, C. Wijmenga, and R.M. Plenge. 2011. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genetics 7, e1002004.
Tsygankov, A.Y. 2013. TULA-family proteins: a new class of cellular regulators. Journal of Cellular Physiology 228: 43–49.
Carpino, N., S. Turner, D. Mekala, Y. Takahashi, H. Zang, T.L. Geiger, P. Doherty, and J.N. Ihle. 2004. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity 20: 37–46.
Hochberg, M.C. 1997. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism 40: 1725.
Gladman, D.D., D. Ibanez, and M.B. Urowitz. 2000. Systemic lupus erythematosus disease activity index. Journal of Rheumatology 29: 288–291.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif) 25: 402–408.
Pan, H.F., J.H. Tao, and D.Q. Ye. 2010. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opinion on Therapeutic Targets 14: 479–484.
Hendriks, W.J., and R. Pulido. 2013. Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochimica et Biophysica Acta 1832: 1673–1696.
San, Luis B., B. Sondgeroth, N. Nassar, and N. Carpino. 2011. Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. The Journal of Biological Chemistry 286: 15943–15954.
Zenewicz, L.A., C. Abraham, R.A. Flavell, and J.H. Cho. 2010. Unraveling the genetics of autoimmunity. Cell 140: 791–797.
Carpino, N., Y. Chen, N. Nassar, and H.W. Oh. 2009. The Sts proteins target tyrosine phosphorylated, ubiquitinated proteins within TCR signaling pathways. Molecular Immunology 46: 3224–3231.
Stanford, S.M., M.F. Maestre, A.M. Campbell, B. Bartok, W.B. Kiosses, D.L. Boyle, H.A. Arnett, T. Mustelin, G.S. Firestein, and N. Bottini. 2013. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: a novel role of SH2 domain-containing phosphatase 2 as a modulator of invasion and survival. Arthritis and Rheumatism 65: 1171–1180.
Feshchenko, E.A., E.V. Smirnova, G. Swaminathan, A.M. Teckchandani, R. Agrawal, H. Band, X. Zhang, R.S. Annan, S.A. Carr, and A.Y. Tsygankov. 2004. TULA: an SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene 23: 4690–4706.
Guerra, S.G., T.J. Vyse, and D.S. Cunninghame Graham. 2012. The genetics of lupus: a functional perspective. Arthritis Research and Therapy 14: 211.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81473058). We want to thank the patients and healthy controls for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Ni, J., Li, LJ. et al. Decreased UBASH3A mRNA Expression Levels in Peripheral Blood Mononuclear Cells from Patients with Systemic Lupus Erythematosus. Inflammation 38, 1903–1910 (2015). https://doi.org/10.1007/s10753-015-0170-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0170-9