Abstract
Three sardine species of Harengula and one of Opisthonema (Clupeiformes, Clupeidae) are known in the Western Atlantic, where the Amazon-Orinoco plume has been recognized as a major biogeographic barrier, albeit permeable to larger and generalist species. Here we used mitochondrial cox1 gene DNA sequences to check the lineage delimitation of both genera, testing the influence of the Amazon-Orinoco barrier (AOB) and marine provinces on their phylogeographic structure. Results indicate that the two genera are differently affected by the AOB, including cryptic speciation in Harengula and population structure in Opisthonema. Harengula show a broad distribution in the Brazilian Province (BRA) distinct from H. clupeola and H. jaguana from the Greater Caribbean Region (GCR). Divergence time between Harengula from the GCR vs. BRA was estimated as about 2.4 Mya, which coincides with the period of increasing sediment and freshwater discharge of the Amazon River in the Atlantic. Results also indicate the existence of a single species of Opisthonema, albeit with population structuring related to the marine provinces. Since species of both genera are relevant to artisanal fisheries and the maintenance of oceanic ecosystems, these results may help in fisheries management of these important marine resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxonomic uncertainties and lack of proper stock delimitation can seriously compromise the management of fisheries resources (Carvalho & Hauser, 1994; Ward et al., 2005). Fisheries statistics are particularly sensitive to species misidentification (FAO, 2016), a situation that is common among pelagic and forage fishes. Additionally, molecular tools are useful in identifying hidden diversity, including species relevant to fisheries (Tourinho et al., 2012; Thomas et al., 2014; Lima et al., 2017). The use of mitochondrial DNA, despite its limitations, has already revealed cryptic species in several commercial fishes (Mat Jaafar et al., 2012; Wu et al., 2016; Durand et al., 2017; Jacobina et al., 2020), resulting in a refined understanding of species distributions and population structure.
Clupeids are among the most economically relevant forage fishes for both artisanal and industrial fisheries worldwide, such as sardines and herrings (Whitehead, 1985; Birge et al., 2021). In addition to serving as food, they are also often used as bait for fishing larger species (Whitehead, 1985; Lopes et al., 2017). They are most often marine and pelagic, but some species exclusively inhabit freshwaters, whereas others are euryhaline or anadromous (Whitehead, 1985). Harengula Valenciennes 1847 and Opisthonema Gill 1861 are two clupeid genera from the Atlantic and Pacific coasts of the New World. Both are represented by small to medium-sized schooling fishes that are usually found along the coast but also in estuaries and lagoons (Whitehead, 1985; Miller et al., 2005; Petry et al., 2016; Pinheiro et al., 2018). Harengula is also distinct in the Southwestern Atlantic since it is the only clupeid in the Brazilian oceanic islands of the Rocas Atoll, and the Trindade-Martin Vaz and Fernando de Noronha archipelagos (Gasparini & Floeter, 2001; Sazima et al., 2006; Pinheiro et al., 2018).
The Atlantic thread herring Opisthonema oglinum (Le Sueur 1818) is the only species of the genus in the Western Atlantic (WA), distributed from the Gulf of Maine in USA to the estuary of la Plata River in Uruguay (Munroe et al., 2015a). In turn, three species of scaled-sardines of the genus Harengula are recognized in the WA: Harengula clupeola (Cuvier 1829), Harengula humeralis (Cuvier 1829), and Harengula jaguana Poey 1865 (Whitehead, 1973, 1985; Fricke et al., 2024). While H. humeralis is reported from the eastern Florida (USA) to the French Guiana (Munroe et al., 2015b) and possibly in the north coast of Brazil (Cervigón, 1991; Robertson & Van Tassell, 2023), H. clupeola and H. jaguana are broadly distributed from the east coast of USA to southern Brazil (Whitehead, 1985; Munroe et al., 2015c, 2019). Harengula humeralis can be easily distinguished from congeners based on morphology (Whitehead, 1985; Cervigón, 1991), but anatomical distinction between H. clupeola and H. jaguana is challenging (Rivas, 1963; Berry, 1964; Whitehead, 1967, 1973, 1985). In the most recent revision of the genus, Whitehead (1985: 66) also indicated the likely existence of one or more subspecies of H. jaguana along its extensive range, as previously suggested by Rivas (1950). This might indicate population structuring or cryptic species in the genus, as extensively detected in several coastal species previously considered as having a wide distribution in the WA (Colborn et al., 2001; Rocha, 2003; Luiz et al., 2012; Rodríguez-Rey et al., 2017; Dias et al., 2019; Petean et al., 2020; Araujo et al., 2022). In Brazil, they are usually identified as H. clupeola, but both species are reported, while in the oceanic islands a single morphotype is known but its identity is uncertain (Figueiredo & Menezes, 1978; Whitehead, 1985).
Genetic structuring of clupeids can be related to temperature, salinity, and depth, considering that these oceanographic features are known to influence other marine taxa with pelagic larvae (Palumbi, 1994; Floeter et al., 2008; Luiz et al., 2012; Stern et al., 2018; Jacobina et al., 2020). In the WA, the Amazon-Orinoco barrier (AOB) marks the limit between the Greater Caribbean biogeographic region (GCR), composed by the Carolinian and the Caribbean Provinces in the north, and the Brazilian Province (BRA) in the south (Floeter et al., 2008; Briggs & Bowen, 2012; Robertson & Cramer, 2014). The AOB is considered a biogeographic filter acting in the genetic structuring of several marine coastal fishes especially since the Pleistocene (Rocha et al., 2002, 2008; Rocha, 2003; Floeter et al., 2008; Luiz et al., 2012; Reis et al., 2016; Jacobina et al., 2020; Araujo et al., 2022; Quintão et al., 2022). The effectiveness of this barrier varied across taxa and with increased sedimentation and sea-level fluctuations through interglacial and glacial periods (Rocha, 2003; Figueiredo et al., 2009; Ludt & Rocha, 2015; Araujo et al., 2022). The AOB is also less effective in the isolation of larger species or those that are more tolerant to variations in salinity (Araujo et al., 2022; Giachini Tosetto et al., 2022).
The effectiveness of a barrier can be estimated by comparing the genetic structure of species with distinct biological attributes and from different marine provinces (Araujo et al., 2022). Herein we tested the role of the AOB in the genetic structure of two sardine genera along the WA based on DNA sequences of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene. Since both Harengula and Opisthonema are pelagic fish, we expect to find a genetic signature of the AOB on them. However, due to the smaller size (common length: 10–12 cm vs. 20 cm standard length, respectively, Whitehead, 1985) and putative subspecies along its distribution, Harengula must be more affected by the AOB and other oceanographic barriers them Opisthonema. Additionally, we provide information regarding taxonomy and fisheries stocks, including the identity of Harengula from Fernando de Noronha oceanic archipelago, which is in the center of a fisheries conflict in the island (Mendes et al., 2020).
Material and methods
DNA extraction, amplification, and sequencing
DNA sequences were obtained from specimens acquired in fish markets, collected using a 5 m-long beach-seine (5 mm mesh) or deposited in fish collections from 19 localities along the Brazilian coast and the Fernando de Noronha oceanic archipelago, off northeastern Brazil (Online Resource 1). Collection of specimens in Fernando de Noronha was conducted under the System Authorization and Information on Biodiversity permit (SISBIO nº 67671-1). Tissue samples were stored in 98% ethanol, and the voucher specimens were fixed in a formaldehyde 4% solution, transferred to a 70% ethanol solution, and then deposited in the ichthyological collections of the Universidade Federal do Rio Grande do Norte (UFRN) and the Instituto de Biodiversidade e Sustentabilidade, Universidade Federal do Rio de Janeiro (NPM). Specimens were morphologically identified at the genus level based on Figueiredo and Menezes (1978) and Whitehead (1985).
Genomic DNA was extracted by saline protocol based on Bruford et al. (1998) with some modifications (Online Resource 2). DNA amplification by PCR was performed using the GoTaq® Green Master mix (Promega, USA) and the primers FISH-BCL (5′- TCAACYAATCAYAAAGATATYGGCAC) and FISH-BCH (5′- TAAACTTCAGGGTGACCAAAAAATCA) of the mitochondrial gene cox1 (Baldwin et al., 2009). PCR steps consisted in a first cycle of 2 min at 95 °C, 35 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 1 min, and a final cycle of 10 min at 72 °C, according to Baldwin et al. (2009). Amplicons were sequenced in both directions by Macrogen Inc (https://dna.macrogen.com/).
Additional sequences of specimens from USA, Mexico, and Caribbean were obtained from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and aligned with our sequences (Online Resource 1). Three datasets were assembled: the first (Harengula dataset) includes sequences of H. clupeola, H. jaguana, and Harengula sp. from the WA, with Sardinella aurita Valenciennes 1847 as outgroup; the second (Opisthonema dataset) includes sequences of Opisthonema from the WA, also with S. aurita as outgroup. Finally, the third dataset (combined dataset) is the merger of the two previously mentioned datasets.
Phylogenetic analysis and lineage delimitation
Forward and reverse sequences of Opisthonema and Harengula were edited, and consensus sequences of 555 bp were defined in SeqTrace v. 0.9 (Stucky, 2012). The sequences obtained were deposited in GenBank (MW302057-MW32121, Online Resource 1). Sequences of the Opisthonema, Harengula, and the combined datasets were separately aligned using the MUSCLE algorithm (Edgar, 2004), and with the best evolutionary models selected in MEGA 11 (Tamura et al., 2021). Following the Bayesian Information Criterion, the evolutionary model used for the combined dataset was Kimura 2-parameter with invariant sites (K2P + I), while for the Opisthonema and Harengula datasets were K2P with gamma distribution (K2P + G).
Bayesian Inference (BI) was performed in BEAST v. 1.10.4 (Suchard et al., 2018) using the following parameters: substitution model as Hasegawa-Kishino-Yano with invariant sites (HKY + I) for the combined dataset, and HKY + G for the Harengula and Opisthonema datasets with base frequencies as all equal (since there is no K2P model in BEAST, the equivalent of it is HKY with base frequencies equal). The selected clock type was strict with normal distribution and mean of 0.01 mutations/Mya, a substitution rate suggested for fish mtDNA (Bermingham et al., 1997; Thomaz et al., 2015), and standard deviation of 0.001. The tree prior model was set as speciation with Yule process for the combined and Harengula datasets and coalescent with constant size for the Opisthonema dataset. The pInv prior (proportion of invariant sites parameter) selected was normal distribution with mean of 0.61 and standard deviation of 0.01 for the combined dataset. The alpha prior (gamma shape parameter) selected was normal distribution with mean of 0.24 and 0.17 and standard deviation of 0.01 for Harengula and Opisthonema datasets, respectively. The Markov chain Monte Carlo (MCMC) simulations were run with 20,000,000 generations and sampled every 2,000 generations in all datasets. Other parameters were set as default. To ensure quality of the MCMC simulations, ESS values of at least 200 were checked using Tracer v. 1.7.1 (Rambaut et al., 2018). TreeAnnotator v. 1.10.2 was used to summarize results of BEAST into a single tree with burn-in of 20% and a posterior probability limit of 0.5. The final trees for each dataset were visualized and edited in FigTree v. 1.4.4 (Rambaut, 2018).
For lineage delimitations, we analyzed the Harengula and Opisthonema datasets using four single-locus methods: multi-rate Poisson Tree Processes (mPTP) (Kapli et al., 2017); single-threshold Generalized Mixed Yule-Coalescent (sGMYC), and multiple-threshold GMYC (mGMYC) (Fujisawa & Barraclough, 2013); and Automatic Barcode Gap Discovery (ABGD) (Puillandre et al., 2012). A Maximum Likelihood tree generated on MEGA 7 (Kumar et al., 2016) with 1,000 replications, Nearest-Neighbor-Interchange with branch swap filter as moderate, was used as input tree for mPTP. The mPTP was performed on an online server (https://mptp.h-its.org/#/tree) using the default parameters. Ultrametric trees generated on BEAST from both the Opisthonema and Harengula datasets were used as input file for sGMYC and mGMYC. Both analyses were performed on an online server (https://species.h-its.org/gmyc/). ABGD distance-based analyses were run through the online server at https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html, with the relative gap width of 1.0 and the remaining parameters set as default for all the distances available (Jukes-Cantor, Kimura, and simple distance). In this analysis, the delineation considered was the one with p-value of 0.01, as suggested by previous studies (Puillandre et al., 2012; Blair & Bryson, 2017). For the delimitation based on genetic distance, the genetic divergence (K2P) was calculated in MEGA 7 and a threshold value was set using the cut-off values of 2% of divergence for cox1 (Ward, 2009).
To detect molecular structuring in Opisthonema and Harengula, we used GENELAND, which does not require a priori assignment of samples (Guillot et al., 2005). The GENELAND analysis was based on an uncorrelated frequency model, which is used to delimit clusters of possible distinct lineages (Pavón-Vázquez et al., 2018), with a minimum population number 1 and maximum population number 10. The spatial model was selected to infer the number of clusters in nine independent runs using 1,000,000 MCMC iterations, of which every 1,000 was retained. A burn-in of 200 was applied and the run with the highest mean logarithm of posterior probability was used to compute the posterior probabilities of population membership. Additionally, a haplotype network was inferred using the TSC method in PopART software (Leigh & Bryant, 2015) to highlight the degree of divergence and spatial distribution of the molecular diversity of each taxa along the three marine provinces. To further investigate population structure, we performed Analyses of Molecular Variance (AMOVA) using Arlequin v. 3.5.2.2 (Excoffier & Lischer, 2010) to test the structuring hypotheses regarding the AOB, the marine provinces and those suggested by GENELAND, for each taxa. Significance was inferred using 1,000 random permutations. Gene flow (ɸST) was also accessed in AMOVA, as the variance among populations within groups.
Results
A total of 33 cox1 sequences of Harengula and 32 of Opisthonema from the BRA were sequenced and edited, then aligned with 61 sequences of H. clupeola, H. jaguana, and Harengula sp., and 32 sequences of O. oglinum available in GenBank. The Bayesian analysis of the combined dataset (Fig. 1) indicated a clearly distinct assemblage formed by all sequences Harengula sp. and H. clupeola from BRA, including individuals from the Fernando de Noronha Archipelago, which is herein referred to as Harengula sp. BRA. This clade is sister of Harengula from the GCR, with most specimens identified as H. clupeola restricted to the Caribbean Province (except for a single Harengula sp. from Florida, USA), and all H. jaguana, most of them from the Carolinian Province (10 in 15) in well-supported clades (posterior probability = 1). The separation between Harengula sp. BRA and the clade formed by H. clupeola and H. jaguana was estimated at approximately 2.5 Mya (3.5–1.5 Mya), suggesting the Amazon-Orinoco plume as a barrier, but also a signature of the marine provinces in the genetic structuring. In Opisthonema, there are few well-supported clades, and the analysis overall failed to reveal a clear biogeographic pattern (Fig. 1), indicating a permeable role of the Amazon-Orinoco plume in this widely distributed species.
Bayesian time-calibrated tree including sequences of Harengula and Opisthonema from the Western Atlantic (combined dataset). Clade colors represent Carolinian Province (blue), Caribbean Province (red), and Brazilian Province (green). Numbers on branches are posterior probability values. Blue bars over nodes are confidence intervals for dates of cladogenetic events. Scale in millions of years (Mya)
Similar results were obtained in the lineage delimitation analyses of Harengula (Fig. 2). The sGMYC, ABGD, and genetic distance (Gdist) also indicated three lineages, two of them formed by H. clupeola and H. jaguana clades from the GCR, and another formed by Harengula sp. BRA (Online Resource 3). All analyses recovered both H. jaguana and Harengula sp. BRA clades. Lastly, sequences of H. clupeola from GCR were subdivided in different lineages in the mPTP and mGMYC analyses (Fig. 2). For the Opisthonema dataset, delimitation analyses were also incongruent among themselves, with mPTP, ABGD, and genetic distance (Gdist) indicating a single lineage. However, the sGMYC and mGMYC analyses subdivided O. oglinum into several lineages that are not concordant with the AOB or the marine provinces (Fig. 2).
Bayesian Inference tree and lineage delimitation analyses of Harengula and Opisthonema from the Western Atlantic. Clade colors represent Carolinian Province (blue), Caribbean Province (red), and Brazilian Province (green). White circles over nodes indicate high posterior probability values (> 0.85). Bars on the right side indicate lineages delimited by the following analyses: mPTP—multiple rate PTP; sGMYC—single-threshold of Generalized Mixed Yule-Coalescent; mGMYC—multiple-threshold GMYC; ABGD—Automatic Barcode Gap Discovery; Gdist—Genetic distance (K2P). Number inside the bars correspond to grouping
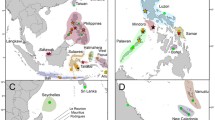
Molecular structuring analyses also recovered three main lineages of Harengula separated by the AOB and marine provinces, one formed by sequences of H. jaguana and H. clupeola from Carolinian Province, other containing H. jaguana and H. clupeola from Caribbean Province, and another restricted to BRA (Fig. 3). In Opisthonema, while three populations were indicated by the analysis, we were not able to visually determine these clusters based on the result map (Fig. 4).
Maps of posterior probabilities of population membership and spatial location of genetic discontinuities in Harengula from the Western Atlantic. Three main clusters (K = 3) can be visualized (d): Harengula clupeola and H. jaguana from Caribbean Province (a), Harengula sp. BRA from the Brazilian Province (b), and H. clupeola and H. jaguana from Carolinian Province (c). Darker colors indicate highest probabilities of membership and contour lines represent the spatial position of genetic discontinuities between lineages
Maps of posterior probabilities of cluster membership and spatial location of genetic discontinuities in Opisthonema oglinum from the Western Atlantic. Three main clusters (K = 3) were recovered (d), but it is not possible to clearly determine their geographical limits. Darker colors indicate highest probabilities of membership and contour lines represent the spatial position of genetic discontinuities between populations
The haplotype network of Harengula showed a deep structure, with 18 mutational steps (4.8% genetic divergence) between Harengula sp. BRA and the group formed by H. clupeola and H. jaguana from GCR. Ten mutational steps (2% genetic divergence) also separate H. clupeola and H. jaguana clades (Fig. 5). Individuals from the Fernando de Noronha Archipelago share the same haplotype from the northeastern Brazilian coast. The haplotype network of Opisthonema oglinum, in turn, failed to reveal any clear genetic structure, except for two haplotypes exclusively from the Bermuda Archipelago (GCR) and from a few localities in BRA.
The AMOVA results for both Harengula and Opisthonema agree with other results. In Harengula, it indicated that both AOB and marine provinces (the same of GENELAND hypothesis) were significantly structuring the genetic portioning, explaining 30% and 27.4% of the variance (Table 1). In Opisthonema, the AOB was not significant, but the marine provinces hypothesis was (12.6%) (Table 1). ɸST were significant in all scenarios for Harengula, but not for Opisthonema (Table 1), suggesting additional structuring within the main lineages, as also indicated by a few lineage delimitation methods.
Considering the size of both taxa, Harengula sp. BRA, H. clupeola (GCR) and H. jaguana (GCR) presented a smaller size and a stronger influence of the AOB, with different species in each side of the barrier, than in Opistonem oglinum, larger and distributed along the WA (Fig. 6).
Estimates of divergence times between the Harengula species and Opisthonema oglinum populations in the Western Atlantic and fluctuations in sea-level in the last 12.5 Mya, in relation to the three stages of increasing sediment and freshwater discharge of the Amazon River (in shades of gray: Phase 1, 9.4–5.6 Mya; Phase 2, 5.6–2.4 Mya; Phase 3, 2.4 Mya-present). Figure modified from Araujo et al. (2022). The red dotted line corresponds to current sea-level; sea-level curve modified from Haq et al. (1987) and Johnson et al. (2006), following the timescale by Hilgen et al. (2012). The yellow stripe indicates the estimated time interval of the emergence of the Amazon River (9.4–9.0 Mya). Mean and 95% high posterior probabilities are indicated by the dots and lines in each species/population comparison, respectively. Timescale in millions of years (Mya)
Discussion
Role of the Amazon-Orinoco barrier
Soft barriers, such as the AOB, can act as a filter to dispersal, which can promote speciation, but at the same time allow occasional crossings that may lead to the establishment of populations on the other side of the barrier or the maintenance of gene flow between both sides (Luiz et al., 2012; Araujo et al., 2022; Giachini Tosetto et al., 2022; Quintão et al., 2022). This situation was evidenced by the different phylogeographic patterns recovered in two sardine genera of the WA, suggesting cryptic speciation in Harengula and population structuring in Opisthonema. Surprisingly, the marine provinces hypotheses were also significant for both taxa (see AMOVA results), indicating additional oceanographic barriers, as in the case of other reef fishes (e.g. Haemulon aurolineatum Cuvier 1830 and Selene setapinnis (Mitchill 1815)) along the WA (Araujo et al., 2022).
The Amazon River became a transcontinental river around 9.4–9 Mya (Gorini et al., 2014; Hoorn et al., 2017), with a substantial increase in freshwater and sediment discharges estimated at around 2.4 Mya (Figueiredo et al., 2009). The estimated divergence time of H. jaguana and H. clupeola clades in the Greater Caribbean Region (GCR) vs. Harengula sp. BRA is at around 3.5–1.5 Mya, coinciding with the stage of increased sediment and freshwater discharge of the Amazon River, when relatively larger and pelagic species were more affected (Araujo et al., 2022). Interestingly, the AOB might also act as a barrier to H. humeralis, likely restricting its distribution to the north of the AOB, however, its occurrence in the north of Brazil is uncertain (Cervigón, 1991; Robertson & Van Tassell, 2023).
The effectiveness of an oceanographic barrier depends on the biology of each species (Rocha, 2003; Luiz et al., 2012; Araujo et al., 2022). Despite both being pelagic and forage fishes, species of Harengula and Opisthonema likely differ in key biological traits that might account for the distinct responses to the AOB. Opisthonema oglinum seems to be less sensitive to lower salinities when compared to species of Harengula (Paramo et al., 2003). Opisthonema oglinum is also a larger species, about 70% larger than H. clupeola and H. jaguana (38 vs. 22.5–21.2 cm total length (TL) respectively; Cervigón et al., 1992; Da Costa et al., 2018), one feature that might also be important since body size is a key predictor to the dispersal capacity across the AOB (Luiz et al., 2012; Araujo et al., 2022). These differences are comparable to those seen in reef fishes during the last 2.4 Mya, the most intense sedimentation and freshwater discharge period, and average maximum TL of 50.6 cm, as in the population structure of O. oglinum, and the intermediary phase of the Amazon River (5.8–2.4 Mya) and 24.2 cm TL, as in the speciation of Harengula (Araujo et al., 2022).
Taxonomic accounts
Our molecular data of O. oglinum is in agreement with the literature in terms of taxonomy and distribution, however in Harengula, it suggests that H. clupeola and H. jaguana, which are closely related, are restricted to the Carolinian and Caribbean Provinces. Meanwhile, in the Brazilian Province, Harengula sp. BRA may represent another species not formally recognized that may be limited by the AOB (Araujo et al. 2022), since both H. clupeola (type locality, Martinique Island) and H. jaguana (type locality, Cuba) were described based on specimens from the GCR (Whitehead, 1985).
Harengula is the only sardine found in the oceanic islands of the Southwestern Atlantic. Perhaps surprisingly at first, our results indicate a single population of Harengula inhabiting the northeastern Brazilian coast and the Fernando de Noronha Archipelago, located about 300 km off the coast. This suggests a higher dispersive potential across distant and deep marine regions or even a conservative molecular marker. Harengula is also known to inhabit the Rocas Atoll and Trindade-Martin Vaz Archipelago, which are located at about 240 and ~ 1,200 km off the coast, respectively (Gasparini & Floeter, 2001; Simon et al., 2013). The Fernando de Noronha Archipelago is part of the Fernando de Noronha Ridge, which also includes the Rocas Atoll and several seamounts along the northern portion of Brazil (Alberoni et al., 2020). An almost continuous series of seamounts is also present between the Trindade-Martin Vaz Archipelago and the central coast of Brazil (Pinheiro et al., 2018). This might explain the occurrence of Harengula in these distant oceanic islands since seamounts are known to act as steppingstones for some species (Pinheiro et al., 2017; Lima et al., 2022; Simon et al., 2022).
Some delimitation analyses (sGMYC and mGMYC) indicated further subdivisions of H. clupeola and H. jaguana, a result that is congruent with previous studies that concluded that GMYC analyses tend to overestimate the number of lineages (Fujisawa & Barraclough, 2013; Hamilton et al., 2014). The phylogeographic structure detected may reflect some phenotypic differences in H. jaguana (six proposed subspecies), and O. oglinum (one) (Rivas, 1950, 1963; Whitehead, 1985). In Brazil, species of Harengula are either identified as H. clupeola or H. jaguana, without the recognition of subspecies. Harengula macrophthalma (Ranzani 1842), described for Brazil, is currently regarded as a junior synonym of H. clupeola (Whitehead, 1985). However, the identity of the species of Harengula in the Brazilian Province remains to be elucidated in further studies, which would ideally include additional molecular markers and morphological tools in a taxonomic context.
Stock delimitation
Our results are also relevant for fisheries management of these sardine species in the Western Atlantic at different levels. In addition to their relevance to artisanal fisheries, there is a complex ongoing conflict involving the artisanal fishing of Harengula in the Fernando de Noronha Archipelago, a Marine Protected Area (Freire & Pauly, 2015; Lopes et al., 2017; Mendes et al., 2020; Pauly et al., 2020). Our results are the first to shed light on the identity and geographic distribution of the species from the Brazilian Exclusive Economic Zone (BEEZ), indicating that Fernando de Noronha specimens belong to a putative undescribed species of Harengula from Brazil.
As climate change keeps intensifying, fisheries in Brazil are also at increasing risk of reducing productivity, which makes it urgent to correctly define and manage fishing stocks (Lam et al., 2020). Herrings and sardines are overall forage and low trophic level fishes, making them key actors in marine coastal ecosystems since they connect primary production and keystone predators (Pikitch et al., 2014). Future management and conservation plans for Opisthonema oglinum and Harengula sp. in BRA can benefit from the fact that these species apparently have a wide distribution in the extensive BEEZ. Additional phylogeographic studies, with more samples, localities, and variable markers, including genomics, must be done to correct delimit the fisheries stocks of Harengula in the BEEZ, as well as its irreplaceable occurrence in the oceanic islands.
Data availability
The edited and aligned DNA sequences used in this study are available on GenBank database under accession codes MW302057-MW302121. Raw DNA sequences can be shared upon request.
References
Alberoni, A. A. L., I. K. Jeck, C. G. Silva & L. C. Torres, 2020. The new Digital Terrain Model (DTM) of the Brazilian Continental Margin: detailed morphology and revised undersea feature names. Geo-Marine Letters 40: 949–964. https://doi.org/10.1007/s00367-019-00606-x.
Araujo, G. S., L. A. Rocha, N. S. Lastrucci, O. J. Luiz, F. Di Dario & S. R. Floeter, 2022. The Amazon-Orinoco Barrier as a driver of reef-fish speciation in the Western Atlantic through time. Journal of Biogeography. https://doi.org/10.1111/jbi.14398.
Baldwin, C. C., J. H. Mounts, D. G. Smith & L. A. Weigt, 2009. Genetic identification and color descriptions of early life-history stages of Belizean Phaeoptyx and Astrapogon (Teleostei: Apogonidae) with Comments on identification of adult Phaeoptyx. Zootaxa 2008: 1–22. https://doi.org/10.11646/zootaxa.2008.1.1.
Bermingham, E., S. S. McCafferty & A. Martin, 1997. Fish biogeography and molecular clocks: perspectives from the Panamanian Isthmus. In Kocher, T. D. & C. A. Stepien (eds), Molecular Systematics of Fishes Academic Press, San Diego: 113–128.
Berry, F. H., 1964. A hypomaxillary bone in Harengula (Pisces: Clupeidae). Pacific Science University of Hawai’i Press XVIII: 373–377.
Birge, T. L., G. M. Ralph, F. Di Dario, T. A. Munroe, R. W. Bullock, S. M. Maxwell, M. D. Santos, H. Hata & K. E. Carpenter, 2021. Global conservation status of the world’s most prominent forage fishes (Teleostei: Clupeiformes). Biological Conservation 253: 108903. https://doi.org/10.1016/j.biocon.2020.108903.
Blair, C. & R. W. Bryson Jr., 2017. Cryptic diversity and discordance in single-locus species delimitation methods within horned lizards (Phrynosomatidae: Phrynosoma). Molecular Ecology Resources 17: 1168–1182. https://doi.org/10.1111/1755-0998.12658.
Briggs, J. C. & B. W. Bowen, 2012. A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography 39: 12–30. https://doi.org/10.1111/j.1365-2699.2011.02613.x.
Bruford, M. W., O. Hanotte, J. F. Y. Brookfield & T. A. Burke, 1998. Multilocus and single-locus DNA fingerprinting. In Hoelzel, C. A. R. (ed), Molecular Genetic Analysis of Populations Oxford University Press: 287–336.
Carvalho, G. R. & L. Hauser, 1994. Molecular genetics and the stock concept in fisheries. Reviews in Fish Biology and Fisheries 4: 326–350. https://doi.org/10.1007/BF00042908.
Cervigón, F., 1991. Los Peces Marinos de Venezuela, vol. I. Fundación Científica Los Roques, Caracas:
Cervigón, F., R. Cipriani, W. Fischer, L. Garibaldi, M. Hendrickx, A. Lemus, R. Márquez, J. Poutiers, G. Robaina, & B. Rodriquez, 1992. Guía de campo de las especies comerciales marinas y de aguas salobres de la costa septentrional de Sur América. Roma.
Colborn, J., R. E. Crabtree, J. B. Shaklee, E. Pfeiler & B. W. Bowen, 2001. The evolutionary enigma of bonefishes (Albula spp.): cryptic species and ancient separations in a globally distributed shorefish. Evolution 55: 807–820. https://doi.org/10.1554/0014-3820(2001)055[0807:TEEOBA]2.0.CO;2.
Da Costa, M. R., R. D. A. Tubino & C. Monteiro-Neto, 2018. Length-based estimates of growth parameters and mortality rates of fish populations from a coastal zone in the Southeastern Brazil. Zoologia 35: 1–8. https://doi.org/10.3897/zoologia.35.e22235.
Dias, R. M., S. M. Lima, L. F. Mendes, D. F. Almeida, P. C. Paiva & M. R. Britto, 2019. Different speciation processes in a cryptobenthic reef fish from the Western Tropical Atlantic. Hydrobiologia 837: 133–147. https://doi.org/10.1007/s10750-019-3966-z.
Durand, J.-D., N. Hubert, K.-N. Shen & P. Borsa, 2017. DNA barcoding grey mullets. Reviews in Fish Biology and Fisheries 27: 233–243. https://doi.org/10.1007/s11160-016-9457-7.
Edgar, R. C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340.
Excoffier, L. & H. E. Lischer, 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567.
FAO, 2016. The State of World Fisheries and Aquaculture, Contributing to food security and nutrition for all, Rome:
Figueiredo, J. L. & N. A. Menezes, 1978. Manual de peixes marinhos do sudeste do Brasil, Museu de Zoologia USP, São Paulo:
Figueiredo, J., C. Hoorn, P. van der Ven & E. Soares, 2009. Late Miocene onset of the Amazon River and the Amazon deep-sea fan: evidence from the Foz do Amazonas Basin. Geology 37: 619–622. https://doi.org/10.1130/G25567A.1.
Floeter, S. R., L. A. Rocha, D. R. Robertson, J. C. Joyeux, W. F. Smith-Vaniz, P. Wirtz, A. J. Edwards, J. P. Barreiros, C. E. L. Ferreira, J. L. Gasparini, A. Brito, J. M. Falcón, B. W. Bowen & G. Bernardi, 2008. Atlantic reef fish biogeography and evolution. Journal of Biogeography 35: 22–47. https://doi.org/10.1111/j.1365-2699.2007.01790.x.
Freire, K. D. M. F., & D. Pauly, 2015. Fisheries Catch Reconstructions for Brazil’s Mainland and Oceanic Islands. Fisheries Centre, University of British Columbia. Retrieved March 24, 2020, from https://doi.library.ubc.ca/10.14288/1.0354313.
Fricke, R., W. N. Eschmeyer, & R. van der Laan, 2024. Eschmeyer’s catalog of fishes: genera, species, references. Retrieved January 13, 2024, from http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Fujisawa, T. & T. G. Barraclough, 2013. Delimiting species using single-locus data and the generalized mixed yule coalescent approach: a revised method and evaluation on simulated data sets. Systematic Biology 62: 707–724. https://doi.org/10.1093/sysbio/syt033.
Gasparini, J. L. & S. R. Floeter, 2001. The shore fishes of Trindade Island, western South Atlantic. Journal of Natural History 35: 1639–1656. https://doi.org/10.1080/002229301317092379.
Giachini Tosetto, E., A. Bertrand, S. Neumann-Leitão & M. Nogueira Júnior, 2022. The Amazon River plume, a barrier to animal dispersal in the Western Tropical Atlantic. Scientific Reports 12: 537. https://doi.org/10.1038/s41598-021-04165-z.
Gorini, C., B. U. Haq, A. T. Dos Reis, C. G. Silva, A. Cruz, E. Soares & D. Grangeon, 2014. Late Neogene sequence stratigraphic evolution of the Foz do Amazonas Basin, Brazil. Terra Nova 26: 179–185. https://doi.org/10.1111/ter.12083.
Guillot, G., F. Mortier & A. Estoup, 2005. GENELAND: a computer package for landscape genetics. Molecular Ecology Notes 5: 712–715. https://doi.org/10.1111/j.1471-8286.2005.01031.x.
Hamilton, C. A., B. E. Hendrixson, M. S. Brewer & J. E. Bond, 2014. An evaluation of sampling effects on multiple DNA barcoding methods leads to an integrative approach for delimiting species: a case study of the North American tarantula genus Aphonopelma (Araneae, Mygalomorphae, Theraphosidae). Molecular Phylogenetics and Evolution 71: 79–93. https://doi.org/10.1016/j.ympev.2013.11.007.
Haq, B. U., J. Hardenbol & P. R. Vail, 1987. Chronology of fluctuating sea levels since the Triassic. Science 235: 1156–1167. https://doi.org/10.1126/science.235.4793.1156.
Hilgen, F. J., L. J. Lourens, J. A. Van Dam, A. G. Beu, A. F. Boyes, R. A. Cooper, W. Krijgsman, J. G. Ogg, W. E. Piller, & D. S. Wilson, 2012. The Neogene Period the Geologic Time Scale (pp. 923–978). Elsevier. https://doi.org/10.1016/B978-0-444-59425-9.00029-9.
Hoorn, C., G. R. Bogotá-A, M. Romero-Baez, E. I. Lammertsma, S. G. A. Flantua, E. L. Dantas, R. Dino, D. A. do Carmo & F. Chemale, 2017. The Amazon at sea: onset and stages of the Amazon River from a marine record, with special reference to Neogene plant turnover in the drainage basin. Global and Planetary Change 153: 51–65. https://doi.org/10.1016/j.gloplacha.2017.02.005.
Jacobina, U. P., R. A. Torres, P. R. A. de Mello Affonso, E. V. dos Santos, L. L. Calado & J. de Araújo Bitencourt, 2020. DNA barcoding reveals cryptic diversity and peculiar phylogeographic patterns in mojarras (Perciformes: Gerreidae) from the Caribbean and South-western Atlantic. Journal of the Marine Biological Association of the United Kingdom. https://doi.org/10.1017/S0025315419001206.
Johnson, W. E., E. Eizirik, J. Pecon-Slattery, W. J. Murphy, A. Antunes, E. Teeling & S. J. O’Brien, 2006. The late Miocene radiation of modern Felidae: a genetic assessment. Science 311: 73–77. https://doi.org/10.1126/science.1122277.
Kapli, P., S. Lutteropp, J. Zhang, K. Kobert, P. Pavlidis, A. Stamatakis & T. Flouri, 2017. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov Chain Monte Carlo. Bioinformatics 33: 1630–1638. https://doi.org/10.1093/bioinformatics/btx025.
Kumar, S., G. Stecher & K. Tamura, 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. https://doi.org/10.1093/molbev/msw054.
Lam, V. W. Y., E. H. Allison, J. D. Bell, J. Blythe, W. W. L. Cheung, T. L. Frölicher, M. A. Gasalla & U. R. Sumaila, 2020. Climate change, tropical fisheries and prospects for sustainable development. Nature Reviews Earth & Environment 1: 440–454. https://doi.org/10.1038/s43017-020-0071-9.
Leigh, J. W. & D. Bryant, 2015. popart: full-feature software for haplotype network construction. Methods in Ecology and Evolution 6: 1110–1116. https://doi.org/10.1111/2041-210X.12410.
Lima, F. D., W. M. Berbel-Filho, T. S. Leite, C. Rosas & S. M. Q. Lima, 2017. Occurrence of Octopus insularis Leite and Haimovici, 2008 in the Tropical Northwestern Atlantic and implications of species misidentification to octopus fisheries management. Marine Biodiversity 47: 723–734. https://doi.org/10.1007/s12526-017-0638-y.
Lima, F. D., T. S. Leite & S. M. Q. Lima, 2022. Seamounts and oceanic currents drive the population structure of Octopus insularis in the Southwest Tropical Atlantic. Aquatic Ecology 56: 1143–1155. https://doi.org/10.1007/s10452-022-09955-9.
Lopes, P. F. M., L. Mendes, V. Fonseca & S. Villasante, 2017. Tourism as a driver of conflicts and changes in fisheries value chains in marine protected areas. Journal of Environmental Management 200: 123–134. https://doi.org/10.1016/j.jenvman.2017.05.080.
Ludt, W. B. & L. A. Rocha, 2015. Shifting seas: the impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa. Journal of Biogeography 42: 25–38. https://doi.org/10.1111/jbi.12416.
Luiz, O. J., J. S. Madin, D. R. Robertson, L. A. Rocha, P. Wirtz & S. R. Floeter, 2012. Ecological traits influencing range expansion across large oceanic dispersal barriers: insights from tropical Atlantic reef fishes. Proceedings of the Royal Society B: Biological Sciences 279: 1033–1040. https://doi.org/10.1098/rspb.2011.1525.
Mat Jaafar, T. N. A., M. I. Taylor, S. A. Mohd Nor, M. de Bruyn & G. R. Carvalho, 2012. DNA barcoding reveals cryptic diversity within commercially exploited Indo-Malay Carangidae (Teleosteii: Perciformes). PLoS ONE 7: e49623. https://doi.org/10.1371/journal.pone.0049623.
Mendes, L. F., P. F. M. Lopes, F. Di Dario, S. M. Q. Lima, J. F. R. Coelho, A. B. A. Bennemann, T. Ferreira-Araújo & F. F. Petean, 2020. O “conflito da sardinha”: a recente liberação da pesca de sardinhas no Parque Nacional Marinho do arquipélago de Fernando de Noronha, Boletim Sociedade Brasileira de Ictiologia:, 6–15.
Miller, R. R., W. L. Minckley & S. M. Norris, 2005. Freshwater fishes of México, University of Chicago Press, Chicago:
Munroe, T., K. A. Aiken, J. Brown, & L. Grijalba Bendeck, 2015a. Opisthonema oglinum. The IUCN Red List of Threatened Species 2015: eT16466100A16509612. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T16466100A16509612.en.
Munroe, T., K. A. Aiken, J. Brown, & L. Grijalba Bendeck, 2015b. Harengula humeralis. The IUCN Red List of Threatened Species 2015: e.T16449708A16510132. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T16449708A16510132.en.
Munroe, T., K. A. Aiken, J. Brown, & L. Grijalba Bendeck, 2015c. Harengula clupeola. The IUCN Red List of Threatened Species 2015: e.T16449654A16510257. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T16449654A16510257.en.
Munroe, T., K. A. Aiken, J. Brown, L. Grijalba Bendeck, & M. Vega-Cendejas, 2019. Harengula jaguana. The IUCN Red List of Threatened Species 2019: e.T190478A86377366. International Union for Conservation of Nature. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T190478A86377366.en.
Palumbi, S. R., 1994. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics 25: 547–572. https://doi.org/10.1146/annurev.es.25.110194.002555.
Paramo, J., R. A. Quiñones, A. Ramirez & R. Wiff, 2003. Relationship between abundance of small pelagic fishes and environmental factors in the Colombian Caribbean Sea: an analysis based on hydroacoustic information. Aquatic Living Resources 16: 239–245. https://doi.org/10.1016/S0990-7440(03)00043-3.
Pauly, D., D. Zeller, & M. L. D. Palomares, 2020. Sea around us concepts, design and data (seaaroundus.org). Retrieved from http://www.seaaroundus.org/
Pavón-Vázquez, C. J., U. O. García-Vázquez, R. W. Bryson, M. Feria-Ortiz, N. L. Manríquez-Morán & A.N.-M. de Oca, 2018. Integrative species delimitation in practice: revealing cryptic lineages within the short-nosed skink Plestiodon brevirostris (Squamata: Scincidae). Molecular Phylogenetics and Evolution 129: 242–257. https://doi.org/10.1016/j.ympev.2018.08.020.
Petean, F. F., G. J. P. Naylor & S. M. Q. Lima, 2020. Integrative taxonomy identifies a new stingray species of the genus Hypanus Rafinesque, 1818 (Dasyatidae, Myliobatiformes), from the Tropical Southwestern Atlantic. Journal of Fish Biology 97: 1120–1142. https://doi.org/10.1111/jfb.14483.
Petry, A. C., T. F. R. Guimarães, F. M. Vasconcellos, S. M. Hartz, F. G. Becker, R. S. Rosa, G. Goyenola, E. P. Caramaschi, J. M. Díaz de Astarloa, L. M. Sarmento-Soares, J. P. Vieira, A. M. Garcia, F. Teixeira de Mello, F. A. G. de Melo, M. Meerhoff, J. L. Attayde, R. F. Menezes, N. Mazzeo & F. Di Dario, 2016. Fish composition and species richness in eastern South American coastal lagoons: additional support for the freshwater ecoregions of the world: fishes in south American coastal lagoons. Journal of Fish Biology 89: 280–314. https://doi.org/10.1111/jfb.13011.
Pikitch, E. K., K. J. Rountos, T. E. Essington, C. Santora, D. Pauly, R. Watson, U. R. Sumaila, P. D. Boersma, I. L. Boyd, D. O. Conover, P. Cury, S. S. Heppell, E. D. Houde, M. Mangel, É. Plagányi, K. Sainsbury, R. S. Steneck, T. M. Geers, N. Gownaris & S. B. Munch, 2014. The global contribution of forage fish to marine fisheries and ecosystems. Fish and Fisheries 15: 43–64. https://doi.org/10.1111/faf.12004.
Pinheiro, H. T., G. Bernardi, T. Simon, J.-C. Joyeux, R. M. Macieira, J. L. Gasparini, C. Rocha & L. A. Rocha, 2017. Island biogeography of marine organisms. Nature 549: 82–85. https://doi.org/10.1038/nature23680.
Pinheiro, H. T., L. A. Rocha, R. M. Macieira, A. Carvalho-Filho, A. B. Anderson, M. G. Bender, F. Di Dario, C. E. L. Ferreira, J. Figueiredo-Filho, R. Francini-Filho, J. L. Gasparini, J.-C. Joyeux, O. J. Luiz, M. M. Mincarone, R. L. Moura, J. A. C. C. de Nunes, J. P. Quimbayo, R. S. Rosa, C. L. S. Sampaio, I. Sazima, T. Simon, D. A. Vila-Nova & S. R. Floeter, 2018. South-western Atlantic reef fishes: zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Diversity and Distributions 24: 951–965. https://doi.org/10.1111/ddi.12729.
Puillandre, N., A. Lambert, S. Brouillet & G. Achaz, 2012. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21: 1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x.
Quintão, T. L., R. Andrades, R. M. Macieira, A. C. Loss & J.-C. Joyeux, 2022. The evolutionary history of Priolepis (Gobiidae) in the Atlantic ocean. Marine Biology 169: 95. https://doi.org/10.1007/s00227-022-04082-3.
Rambaut, A., A. J. Drummond, D. Xie, G. Baele & M. A. Suchard, 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. https://doi.org/10.1093/sysbio/syy032.
Rambaut, A., 2018. FigTree. Retrieved from http://tree.bio.ed.ac.uk/software/figtree/
Reis, R. E., J. S. Albert, F. Di Dario, M. M. Mincarone, P. Petry & L. A. Rocha, 2016. Fish biodiversity and conservation in South America. Journal of Fish Biology 89: 12–47. https://doi.org/10.1111/jfb.13016.
Rivas, L. R., 1950. A Revision of the American Clupeid Fishes of the Genus Harengula with Descriptions of Four New Subspecies. Proceedings of the United States National Museum 100: 275–309.
Rivas, L. R., 1963. Genus Harengula Cuvier and Valenciennes 1847 Fishes of the Western North Atlantic, part 3. Sears Foundation for Marine Research, New Haven: 386–396. https://doi.org/10.5962/bhl.title.7464
Robertson, D. R., & J. Van Tassell, 2023. Shorefishes of the Greater Caribbean: online information system. Smithsonian Tropical Research Institute, Balboa, Panamá. Retrieved from https://biogeodb.stri.si.edu/caribbean/en/pages
Robertson, D. R. & K. L. Cramer, 2014. Defining and dividing the greater Caribbean: insights from the biogeography of shorefishes. PLoS ONE 9: e102918. https://doi.org/10.1371/journal.pone.0102918.
Rocha, L. A., 2003. Patterns of distribution and processes of speciation in Brazilian reef fishes. Journal of Biogeography 30: 1161–1171. https://doi.org/10.1046/j.1365-2699.2003.00900.x.
Rocha, L. A., A. L. Bass, D. R. Robertson & B. W. Bowen, 2002. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeon fishes (Teleostei: Acanthuridae). Molecular Ecology 11: 243–251. https://doi.org/10.1046/j.0962-1083.2001.01431.x.
Rocha, L. A., K. C. Lindeman, C. R. Rocha & H. A. Lessios, 2008. Historical biogeography and speciation in the reef fish genus Haemulon (Teleostei: Haemulidae). Molecular Phylogenetics and Evolution 48: 918–928. https://doi.org/10.1016/j.ympev.2008.05.024.
Rodríguez-Rey, G. T., A. Carvalho Filho, M. E. De Araújo & A. M. Solé-Cava, 2017. Evolutionary history of Bathygobius (Perciformes: Gobiidae) in the Atlantic biogeographic provinces: a new endemic species and old mitochondrial lineages. Zoological Journal of the Linnean Society 182: 360–384. https://doi.org/10.1093/zoolinnean/zlx026.
Sazima, I., C. Sazima & J. M. da Silva-Jr, 2006. Fishes associated with spinner dolphins at Fernando de Noronha Archipelago, tropical Western Atlantic: an update and overview. Neotropical Ichthyology 4: 451–455. https://doi.org/10.1590/S1679-62252006000400009.
Simon, T., R. M. Macieira & J.-C. Joyeux, 2013. The shore fishes of the Trindade–Martin Vaz insular complex: an update. Journal of Fish Biology 82: 2113–2127. https://doi.org/10.1111/jfb.12126.
Simon, T., H. T. Pinheiro, S. Santos, R. M. Macieira, Y. S. S. Ferreira, G. Bernardi, L. A. Rocha, S. R. Floeter, C. E. L. Ferreira & J.-C. Joyeux, 2022. Comparative phylogeography of reef fishes indicates seamounts as stepping stones for dispersal and diversification. Coral Reefs 41: 551–561. https://doi.org/10.1007/s00338-021-02178-8.
Stern, N., J. Douek, M. Goren & B. Rinkevich, 2018. With no gap to mind: a shallow genealogy within the world’s most widespread small pelagic fish. Ecography 41: 491–504. https://doi.org/10.1111/ecog.02755.
Stucky, B. J., 2012. SeqTrace: a graphical tool for rapidly processing DNA sequencing chromatograms. Journal of Biomolecular Techniques 23: 90–93. https://doi.org/10.7171/jbt.12-2303-004.
Suchard, M. A., P. Lemey, G. Baele, D. L. Ayres, A. J. Drummond & A. Rambaut, 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution. https://doi.org/10.1093/ve/vey016.
Tamura, K., G. Stecher & S. Kumar, 2021. MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution 38: 3022–3027. https://doi.org/10.1093/molbev/msab120.
Thomas, R. C., D. A. Willette, K. E. Carpenter & M. D. Santos, 2014. Hidden diversity in sardines: genetic and morphological evidence for cryptic species in the goldstripe sardinella, Sardinella gibbosa (Bleeker, 1849). PLoS ONE 9: e84719. https://doi.org/10.1371/journal.pone.0084719.
Thomaz, A. T., L. R. Malabarba, S. L. Bonatto & L. L. Knowles, 2015. Testing the effect of palaeodrainages versus habitat stability on genetic divergence in riverine systems: study of a Neotropical fish of the Brazilian coastal Atlantic Forest. Journal of Biogeography 42: 2389–2401. https://doi.org/10.1111/jbi.12597.
Tourinho, J. L., A. M. Solé-Cava & C. Lazoski, 2012. Cryptic species within the commercially most important lobster in the tropical Atlantic, the spiny lobster Panulirus argus. Marine Biology 159: 1897–1906. https://doi.org/10.1007/s00227-012-1977-7.
Ward, R. D., 2009. DNA barcode divergence among species and genera of birds and fishes. Molecular Ecology Resources 9: 1077–1085. https://doi.org/10.1111/j.1755-0998.2009.02541.x.
Ward, R. D., T. S. Zemlak, B. H. Innes, P. R. Last & P. D. N. Hebert, 2005. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1847–1857. https://doi.org/10.1098/rstb.2005.1716.
Whitehead, P. J. P., 1967. The clupeoid fishes described by Lacepède, Cuvier and Valenciennes. Bulletin of the British Museum (natural history). Zoology 2: 1–180. https://doi.org/10.5962/p.119073.
Whitehead, P. J. P., 1973. The clupeoid fishes of the Guianas. Bulletin of the British Museum (natural history). Zoology. Supplement. Supplement 5: 1–227. https://doi.org/10.5962/p.119072.
Whitehead, P. J. P., 1985. Clupeoid Fishes of the World (suborder Clupeoidei): an Annotated and Illustrated Catalogue of the Herrings, Sardines, Pilchards, Sprats, Shads, Anchovies, and Wolfherrings, Food and Agriculture Organization of the United Nations, Rome:
Wu, T. H., L. M. Tsang, I.-S. Chen & K. H. Chu, 2016. Multilocus approach reveals cryptic lineages in the goby Rhinogobius duospilus in Hong Kong streams: role of paleodrainage systems in shaping marked population differentiation in a city. Molecular Phylogenetics and Evolution 104: 112–122. https://doi.org/10.1016/j.ympev.2016.07.014.
Acknowledgements
This paper is dedicated to all Brazilian scientists, especially students, who continue to invest in the scientific enterprise in challenging and economically depleted times. We are grateful to J. C. Marenga (Fernando de Noronha) and Adaiso Pereira Silva “Mineiro” (Espírito Santo) for providing Harengula and Opisthonema specimens. We also thank members of RAINHAS (Raias & Sardinhas Research Group), Tiego Costa (IDEMA), and Flávia F. Petean (INTECH—CONICET/UNSAM) for participating in fieldwork in Fernando de Noronha Archipelago. Ricardo Marques and Felipe Magalhães (UFRN) kindly helped in the implementation of phylogenetic analyses. Luciano Barros-Neto assisted in the troubleshooting of population structure analyses. Gabriel S. Araujo provided assistance on the visualization of size and divergence time between lineages. This study is part of the MSc Dissertation of the first author. As members of the evaluation committee, Uedson Jacobina (UFAL) and Marina V. Loeb (MZUSP) provided valuable comments that significantly improved the study. We warmly thank the ReGeneC—Red de Genética para la Conservación for supporting the intellectual development of many young researchers in the conservation genetics field across Latin America.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This study was developed in the context of the "Projeto MULTIPESCA- Ciência para a sustentabilidade da pesca, pescado e pescadores do Rio de Janeiro", which received support from the "Marine and Fisheries Research Project". The Marine and Fisheries Research Project is an offset measure established under a consent decree agreed between the company PRIO and the Federal Public Prosecutors’ Office in Rio de Janeiro. It is implemented by FUNBIO. SMQL thanks Conselho Nacional de Desenvolvimento Científico e Teconológico (CNPq) for the productivity Grant (312066/2021-0). RMM also thanks CNPq for a post-doctoral fellowship (PDS—164822/2020-8). FDD is supported by CNPq PROTAX (443302/2020) and FAPERJ (E-26/210.290/2021). Financial support to JLG was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
TFA: Conceptualization, Formal analysis, Investigation, Resources, Data curation, Writing—original draft, Writing—review & editing, Visualization, Funding acquisition. PHC: Conceptualization, Resources, Writing—review & editing, Supervision, Funding acquisition. FDD: Resources, Writing—review & editing. LFM: Resources, Writing—review & editing. CO: Resources, Writing—review & editing. JLG: Resources, Writing—review & editing. MMR: Resources, Writing—review & editing. RMM: Resources, Writing—review & editing. SMQL: Conceptualization, Resources, Writing—original draft, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to declare that are relevant to the content of this article.
Additional information
Handling editor: Christian Sturmbauer
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferreira-Araújo, T., Hollanda-Carvalho, P., Di Dario, F. et al. Different roles of the Amazon-Orinoco barrier on the genetic structure of two sardine genera from the Western Atlantic Ocean. Hydrobiologia 851, 2429–2445 (2024). https://doi.org/10.1007/s10750-023-05468-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05468-0