Abstract
This study aimed to determine salinity tolerances in Coxiella gastropods from Australian salt lakes and whether different species exhibit characteristically different tolerances. Controlled gradual accumulation experiments were conducted to estimate both the maximum and minimum salinity levels at which 50% of individuals (IC50) remained active for 25 populations representing six species. All studied species showed remarkable euryhalinity and were tolerant of very high levels of salinity, some more than others, while minimum salinity tolerance varied little among populations and species. The experimental trends in salinity tolerances were consistent with the salinity distributions of species in the field, although the former were typically broader than latter. The findings suggest that Coxiella comprises some of the most salt tolerant gastropods globally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of a species from microhabitat to large geographical areas is influenced by a range of factors, including its physiological capabilities (Gaston, 2003). Experimentally determining the physiological tolerances of a species provides information about its fundamental niche, defined as the total range of abiotic conditions that influence the physiology of a species (Devictor et al., 2010). Comparing experimentally determined tolerances with the realised niche, i.e. the actual observed range in which that organism occurs in the field, allows for an understanding of the main factors constraining a species’ distribution and can be used to predict a species response to environmental change (Bozinovic et al., 2011).
A key biological challenge associated with living in an aqueous environment is osmoregulation (Bradley, 2009) as all aquatic organisms must be able to cope with osmotic stress (Kosicka et al., 2020). Salinity tolerance is difficult to define but the working definition used here is the minimum and maximum levels of osmotic pressure from the external environment that an organism can withstand before its internal cells burst or desiccate under hypoosmotic or hyperosmotic conditions, respectively (Deaton, 2009). Much work has been done on estimating the effect of salinity on freshwater or halotolerant taxa (sensu Lawrie et al., 2021), especially in the past 20 years because of concerns about the increasing salinisation of freshwater ecosystems across the globe (Cañedo-Argüelles, 2020). The results suggest that these taxa are often severely impacted by even slight salinity increases (Cañedo-Argüelles, 2020), although some taxa are more tolerant than others (Kefford et al., 2004b, 2012).
The salinity tolerances of halophilic taxa (sensu Lawrie et al., 2021) from salt lakes (enclosed bodies of water with salinity > 3 g/L; Williams, 1964) are also of interest in part because these organisms are salt-adapted and some species are capable of surviving extreme salinities (Hammer, 1986). Salinity can vary considerably within and especially between lakes and over a broad scale appears to be a key driver of community structure in salt lakes (Williams et al., 1990; McEvoy & Goonan, 2003). Understanding the salinity tolerances of halophiles is also becoming important from a conservation perspective because the salinity of salt lakes is increasing due to the increased rates of evaporation and regionalised reductions in rainfall associated with climate change (Williams, 1998; Saccò et al., 2021). Experimental estimates of salinity tolerance are available for a range of halophilic taxa, including gastropods (Davis, 1981; Williams & Mellor, 1991; Filippov & Komendantov, 1996), water beetles (Dytiscidae) (Céspedes et al., 2013), water boatmen (Corixidae) (Carbonell et al., 2012) and crustaceans (Croghan, 1958; Ellis & Williams, 1970; Geddes, 1981; Ismail et al., 2010). The results suggest that halophilic organisms typically display extraordinary euryhalinity, although eventually their salinity tolerances are exceeded resulting in mortality. However, there are many gaps in our knowledge, and it would be useful to determine how tolerances vary among closely related taxa.
Globally very few gastropods inhabit salt lakes and few salt lakes contain gastropods (Hammer, 1986). However, two of the three Tomichiidae genera (Salvador et al., 2022), Tomichia and Coxiella occur in salt lakes (Davis, 1981; Williams & Mellor, 1991). The former consists of seven described species from South Africa (Brown, 1994), which collectively occur in a broad range of habitats and includes two species (Tomichia ventricosa (Reeve, 1842) and Tomichia tristis (Morelet, 1889)) that occur solely or partly in saline vleis (shallow lakes; Davis, 1981). Coxiella contains 15 species, all of which are endemic to Australia (Lawrie et al., 2023). The genus is highly unusual because all species occur in salt lakes and most are only found in these lakes (Lawrie et al., 2021, 2023). Collectively, Coxiella species are widespread and common in permanent and seasonal salt lakes in southern Australia, including Tasmania, although most species are endemic to southern Western Australia (Lawrie et al., 2023). Coxiella and Tomichia are probably Gondwanaland relics (Davis, 1981; Kameda & Kato, 2011). Coxiella appears to have undergone a radiation within Australian salt lakes however, it is unclear whether this radiation has been accompanied by physiological or ecological diversification, e.g. in association with lakes with different salinity profiles.
Current field data suggest that collectively Coxiella species occur over a wide salinity range (0.3–130 g/L) (Geddes et al., 1981; Timms, 1983; Williams et al., 1990; Pinder et al., 2005). However, there is little reliable published information about the salinity distributions of different Coxiella species, especially in Western Australia, in part due to confusion in species identifications (Pinder et al., 2002; Timms, 2009a). Field observations of Coxiella from South Australia suggest that some species (Coxiella glauerti Macpherson, 1957) have higher salinity tolerances than others (Coxiella striata (Reeve, 1842)) (Timms, 2009b; Timms et al., 2014).
The salinity tolerances of one Coxiella species, C. striata, have been experimentally tested. Results from Williams & Mellor (1991) suggested that C. striata is an osmoconformer and that 50% of the individuals could tolerate salinities between 3.8 and 125.5 mS/cm. O’Dwyer & Murphy (2021) suggested that individuals of C. striata from ‘constant’ environments (permanent water and stable salinity) tend to have lower survival under both increasing salinity and temperature stress compared to those individuals from salt lakes that were environmentally unstable (ephemeral and fluctuating salinity). Whether these results are applicable to other Coxiella species is not known and no experiments comparing the salinity response of multiple species have been conducted. From a conservation perspective, understanding how salinity tolerances vary amongst Coxiella species is needed to identify those taxa that are potentially most vulnerable to changing field salinities. The data will also be useful for understanding whether the evolutionary radiation of this group was accompanied by ecological diversification.
This study experimentally tested the upper and lower salinity tolerances of individuals from multiple populations in six Coxiella species. The hypothesis is that salinity tolerance is unequal amongst Coxiella species. These results are compared with field records to understand how well the experimental results correspond with the salinity distributions of these species in nature.
Materials and methods
Sample collections
The experiment used individuals of Coxiella collected between September and December in 2021 from a total of 25 sites (hereafter called populations), representing a total of six species (Fig. 1; Table 1). The six species were selected on the basis that they have overlapping geographic distributions and field records suggest that three of them (Coxiella exposita (Iredale, 1943), Coxiella glabra Macpherson, 1957, Coxiella striatula (Menke, 1843)) are generally found at lower salinities than the other three (Coxiella pyrrhostoma (Cox 1868), C. glauerti and C. n. sp. 2). The number of populations tested per species ranged from two to six (Table 1) and was mainly determined by the number of known populations with enough snails to run the experiments. For some populations, repeated collections were needed to obtain sufficient snails (Table 1). Where possible, populations from throughout the known distribution of each species were represented in the experiments to help document the extent of any intraspecific variation in salinity tolerance. Snails were collected from each site by hand or sieving mud, transported back to the laboratory and placed in collection tanks.
Holding conditions
The collection tanks were held in a temperature-controlled room at 20 °C ± 1 °C, with a 12-h light/dark regime. This temperature was chosen because experimental data of Williams & Mellor (1991) suggested that C. striata had 100% survival over 5 days at this temperature and 20 °C is well within the range of temperatures at which Coxiella is active in the field (Supplemental data). The tanks were aerated and maintained at 39–53 mS/cm with a combination of reverse osmosis (RO) water and Red Sea Salt. This salinity range was chosen because it was well within the tolerable range for all species as indicated by field records (Supplemental data). Since ionic ratios of Australian salt lakes are typically similar to those of the ocean, with only a few exceptions (Bayly & Williams, 1966; Geddes et al., 1981), Red Sea Salt, which is designed to replicate marine water, was used in these experiments. This product has previously been used in experiments with C. striata (O’Dwyer & Murphy, 2021). Snails were fed weekly using Tetra Fin flakes and API algal wafers. Snails were acclimatised to the conditions in the collection tanks for between two and seven days before being included in the experiment.
Experimental design
The experiment tested the response of snails from each population to progressive increases or decreases in salinity. Each experiment had two treatments increasing salinity (‘upper’) and decreasing salinity (‘lower’) and a control (constant salinity). For each population, 30 snails were randomly assigned to each treatment and to the control. The experiment was conducted in 350 mL plastic boxes. Thirty snails from the same population in the same treatment were held in the same box, but snails for each population and each treatment were held in separate boxes.
Each treatment and the control started at a salinity of 53 mS/cm (± 7 mS/cm), with daily water changes used to increase salinity in the upper treatment or reduce salinity in the lower treatment or keep the salinity in the control at 53 mS/cm (± 7 mS/cm). This salinity was selected to minimise the osmotic shock to individuals after their transfer from the collection tanks. The salinity of treatment tanks was changed gradually to replicate salinity changes that salt lakes experience due to evapoconcentration (increasing salinity) or rainfall events (decreasing salinity). Salinity was increased or decreased by an average of 6.3 and 6.8 mS/cm per 24 h, respectively. Below ~9 mS/cm in the lower treatment, conductivities of ~5 mS/cm, ~2 mS/cm and ~0.1 mS/cm were tested. All conductivity data presented here have been corrected to 25 °C.
During the experiment, snails were held at each salinity level for a 24-h period on the basis that it took 20–28 h for the body tissues of a congeneric species (C. striata) to become isosmotic with the surrounding water when specimens were switched from high to low salinity conditions or vice versa (Williams & Mellor, 1991).
To estimate experimental variability, the experiment was repeated three times with fresh sets of snails in each repeat. All populations and treatments within a single experimental run were assayed at the same time. The different experimental runs were conducted at different times between October 2021 and January 2022. Each repeat was started within one to two weeks of the end of the previous one. Where possible, every population was included in every repeat, but only two repeats were possible for CR2, TS2, WH6, LM1, and E33 due to insufficient snails. Similarly, there were not enough snails to include the lower treatment for W3 in the third run.
Field collections
Data on the conductivity of selected field sites inhabited by the six Coxiella study species are presented for comparison with the experimental results (see Supplemental data). Conductivity at these sites was measured using a YSI probe. Whether the snails at the site were active (moving) or inactive (opercula shut) was also recorded. The data were collected between 2017 and 2021 and include repeated measurements from some lakes (see Supplemental data). The identities of the species collected at these sites have been confirmed via genetic data in Lawrie et al. (2023)
Data analysis
The point at which snails became inactive (sealed themselves into their shells by closing their operculum) was measured because once retracted it was considered unlikely for an individual to regain mobility and therefore this point represented the salinity at which individuals could no longer biologically function. Estimates and associated 95% confidence intervals of the salinity at which 50% of tested individuals became inactive (hereafter called IC50) were calculated using the R package medrm, using the metadrm function, which conducts a two-stage meta-analysis to fit a hierarchical dose–response model (Gerhard & Ritz, 2015). This package combines the automated nonlinear regression modelling framework of the package drc (Ritz et al., 2015) with the nonlinear mixed estimation framework of the package nlme (Pinheiro et al., 2015) to produce dose–response estimates for repeated measures experimental designs. IC50 estimates were calculated using a log logistic model for binomial data with curves grouped by population using data from the repeated experimental runs, with the dependant variable ‘inactive’ (i.e. the proportion of inactive/dead specimens at a salinity level) explained by the independent variable ‘salinity’ (mS/cm) and with the correlation between repeated observations from the same experimental unit factored into the model. This modelling was done independently on the lower and upper tolerance datasets. A Kruskal–Wallis test (Hollander et al., 1973) was used to test for a significant difference in the median IC50s for each species for the lower and again for the upper salinity experiments, with post-hoc pairwise comparisons examined using Dunn’s test (Dunn, 1964) and the Bonferroni method used to adjust P-values for multiple comparisons (Hochberg, 1988). All statistical significance was determined using an alpha of P < 0.05.
Results
Controls
Most snails (80% or more) remained active in 68% of the controls (Supplemental data). The lowest percentage of active snails in any control was 50% for population V3 at the end of the 2nd run (Supplementary data).
Salinity range
The salinity ranges for all populations of every species were broad, with high levels of activity observed between 10 and 75 mS/cm (Fig. 2). The minimum salinity at which at least one individual was active was broadly similar for all populations, both within and between species, ranging from 0.1 to 5.7 mS/cm (Table 2). The extent of the variation in the upper salinity limit among the populations of a species ranged from 2.1 mS/cm in C. glauerti to 25 mS/cm in C. glabra (Table 2). The population most tolerant of high salinity was E23 of C. n. sp. 2, which had active individuals at 157.2 mS/cm. In contrast, the highest value recorded for the least tolerant population, i.e. population W3 in C. exposita, was 118.8 mS/cm (Table 2).
Line plot of the percentage of active individuals for each Coxiella species in each run across the measured salinity range. Vertical black bar indicates starting salinity for both the lower and upper experiment with data on the left and right from the lower and upper treatments, respectively. Results for first (solid), second (dashed) and third (dotted) runs are indicated by different line types
Salinity tolerance—variation within species
Different populations in four of the six Coxiella species (C. exposita, C. striatula, C. glauerti and C. n. sp. 2) showed similar upper IC50 estimates and broadly overlapping confidence limits (Fig. 3; Table 2). However, C. glabra and C. pyrrhostoma showed more variation. In the case of C. glabra, the upper IC50 estimates for TS2 (107.8 mS/cm) and MOR2 (104.9 mS/cm) were relatively high compared to those for G1 (87.3 mS/cm), WH6 (88.3 mS/cm) and LM1 (91 mS/cm); the estimate for WH1 (97.3 mS/cm) was intermediate between these two groups (Fig. 3; Table 2). Nevertheless, there was overlap in the 95% confidence limits for all populations of this species except for G1 vs TS2. For C. pyrrhostoma, individuals from E3 (133.4 mS/cm), E4 (131.7 mS/cm) and STR (129.7 mS/cm) had higher upper IC50 estimates than those from E21 (116 mS/cm) and MA5 (116.3 mS/cm; Fig. 3; Table 2).
Different populations in four of the six species (C. exposita, C. glabra, C. striatula, and C. glauerti) displayed almost no difference in their IC50 responses to decreasing salinity (Fig. 3). However, this was not the case for C. pyrrhostoma for which the lower IC50 estimate for the STR population (12.3 mS/cm) was higher than that for the other populations (Fig. 3; Table 2). Also, in C. n. sp. 2, the lower IC50 estimate for E7 population was noticeably higher than that for the E23 but their 95% confidence limits still overlapped (Fig. 3; Table 2).
Salinity tolerance—variation between species
The experiment provided evidence of significant differences in the upper salinity tolerance of some Coxiella species (χ2 = 21.48, df = 5, p-value < 0.001). Estimates of the upper IC50 limits for C. exposita (median = 95.5 mS/cm) and C. glabra (median = 94.1 mS/cm) were significantly different than those for both C. glauerti (median = 138.3 mS/cm) and C. n. sp. 2 (median = 146.4 mS/cm; Table 3; Fig. 3). The upper salinity tolerances of C. striatula (median = 106.3 mS/cm) and C. pyrrhostoma (median = 129.7 mS/cm) were intermediate between these two species groups, with C. striatula more closely aligned with the low salinity species and C. pyrrhostoma having some populations more closely aligned with the low salinity species (MA5, IC50 = 116.3 mS/cm; E21, IC50 = 116 mS/cm) and others with the high salinity species (E3, IC50 = 133.4 mS/cm; E4, IC50 = 131.7 mS/cm; STR, IC50 = 129.7 mS/cm; Table 3; Fig. 3). The maximum upper IC50 values recorded for a species’ population increased in the following order—C. exposita (W3, 99 mS/cm), C. glabra (TS2, 107.8 mS/cm), C. striatula (F1, 113 mS/cm), C. pyrrhostoma (E3, 133.4 mS/cm), C. glauerti (E8, 140.6 mS/cm) to C. n. sp. 2 (E23, 146.5 mS/cm; Fig. 3; Table 2).
The magnitude of difference between species median lower IC50 estimates was not as large compared to the upper salinity tolerances, but some species still demonstrated significantly different tolerances (χ2 = 20.12, df = 5, P = 0.001). Coxiella glauerti (median = 0.3 mS/cm) and C. glabra (median = 0.6 mS/m) were the most tolerant of low salinities and recorded IC50 values of < 1 mS/cm (Fig. 3; Table 2). Coxiella n. sp. 2 was the least tolerant to low salinity (median = 8.6 mS/cm) and showed significantly less tolerance than C. glabra and C. glauerti (Table 3; Fig. 3). Coxiella pyrrhostoma also displayed significantly less tolerance to low salinities than C. glabra and C. glauerti (Table 3).
Field vs experimental data
A total of 151 field records of salinity from 68 different lakes were available for analysis; 136 of these records were for active snails and the remainder for inactive ones (Supplemental data). Most records, 37, 35 and 33, were for C. striatula, C. glabra and C. pyrrhostoma, respectively, while C. n. sp. 2 and C. glauerti had the fewest with 8 and 14, respectively (Fig. 4). Overall, active specimens for each of the six species were recorded from a very broad salinity range, i.e. from 1 to 132 mS/cm (Fig. 4).
Field records for active (red circles) and inactive (blue triangles) individuals from all experimentally tested Coxiella species. Black rectangle is the median salinity of active occurrence (mS/cm). Dark green and purple bars are the lowest and highest 95% confidence intervals for CR2 (C. exposita), WH6 (C. glabra), JB3 (C. striatula), MA5 (C. pyrrhostoma), E22 (C. glauerti) and E23 (C. n. sp. 2). These populations were selected as they each had the lowest upper IC50 for each of their respective species (see Table 2). Note that Fig. 4 contains all water quality data from all available sites, not just the sites from which individuals were collected for the experiment in Table 1
The patterns of active species’ salinity distributions in the field corresponded to those observed in the tolerance experiments. For example, C. n. sp. 2, C. glauerti and C. pyrrhostoma were active in salt lakes that were consistently more saline than those occupied by C. exposita, C. glabra and C. striatula (Fig. 4). Also, as in the experimental results, there was considerable overlap in the field salinities of C. exposita, C. glabra and C. striatula and, among these species, C. exposita was generally found at the lowest salinities (median = 19.1 mS/cm) and C. striatula at the highest (median = 39.3 mS/cm). The field data suggest that C. pyrrhostoma has the broadest salinity range (5.1–132 mS/cm; median = 78.5 mS/cm), notwithstanding that field data for C. glauerti and C. n. sp. 2 are limited. Coxiella n. sp. 2 is yet to be recorded in salinities < 67.9 mS/cm in the field (Fig. 4), which is noteworthy since this species was least tolerant of low salinities in the experiment.
The highest recorded field salinities for active individuals were lower than the experimentally determined upper IC50 estimates for all tested populations in all species except C. pyrrhostoma, for which the four highest field records fall within the estimated 95% confidence interval of the population least tolerant of high salinities (E21, Fig. 4). In addition, inactive individuals were observed in the field at salinities less than or within the IC50 range estimates for populations of C. exposita, C. striatula, C. pyrrhostoma and C. glauerti (Fig. 4). The lowest recorded field salinities were higher than experimentally determined lower IC50 estimates for all tested populations in all species except C. exposita and C. pyrrhostoma (Fig. 4).
Environmental influence
For all but one Coxiella species, there was no relationship between either the upper or lower experimental tolerances and habitat salinity when experimental snails were collected (Fig. 5a, b). However, for the three populations of C. exposita, estimates of the upper salinity tolerance increased with habitat salinity at the time of collection (Fig. 5b).
Scatter plot illustrating the relationship between IC50 estimates and site salinity at the time when the experimental snails were collected for 25 populations of six Coxiella species. a minimum field salinity versus lower IC50 estimate, and b maximum field salinity versus upper IC50 estimate, for each population. Field salinity data are only included when active snails were collected
Discussion
Halophilic gastropods
This study has generated experimental data on the upper and lower salinity tolerances for 25 populations of six Coxiella species. Prior to this, data on salinity tolerance in Coxiella were only available for five populations of one species, C. striata (Williams & Mellor, 1991; O'Dwyer & Murphy, 2021). In the current study, the experimental data were supported by detailed information on the salinity distributions of each species in the field. The results suggest that Coxiella species are amongst the most euryhaline and salt tolerant gastropods in the world, notwithstanding the difficulties of comparing estimates across studies that have used different experimental methods. Field records suggest halophilic gastropods are rare but include Caspiohydrobia spp. from the Aral and Caspian seas (Filippov & Riedel, 2009; Andreeva et al., 2020), Tomichia ventricosa and T. tristis that inhabit saline vleis in South Africa (Davis, 1981) and Heleobia spp. that occur in a range of saline environments in South America (Reid et al., 2021). Of these, salinity tolerances have only been experimentally tested in T. ventricosa, which can remain active between 0 and 50 ppt for a month (Davis, 1981) and Caspiohydrobia spp. (species unknown), which had ~35% survival in tested individuals for two weeks at 110 g/L (Filippov & Komendantov, 1996).
Within species variation
The experimentally determined lower salinity limits varied little among conspecific populations, except that these limits were unusually high for single populations of C. n. sp. 2 and C. pyrrhostoma. The majority of species also did not show significant variation in the upper salinity tolerances among their populations. The notable exceptions were populations of C. pyrrhostoma and of C. glabra which sometimes displayed relatively large differences in maximum salinity tolerance. The increased variability observed in these two species may reflect the fact that they were represented in the experiment by, respectively, five and six populations compared to only two to four populations for C. n. sp. 2, C. exposita and C. glauerti. On the other hand, C. striatula was represented by six populations but showed little intraspecific variation. Comparing the same number of populations for each species would have been ideal, however, this was not possible due to the small number of known populations of C. n. sp. 2, the limited number of Western Australian populations of C. glauerti and a low abundance of snails in known populations of C. exposita.
O’Dwyer & Murphy (2021) compared the stress tolerance of populations of C. striata in lakes with less environmental variance (e.g. permanent water bodies with stable salinities) with those in lakes that experience variable conditions (e.g. ephemeral water bodies with fluctuating salinities). They concluded that the former was less tolerant of environmental change due to stabilising selection while the latter have maintained standing variation in physiological tolerances. The relevance of these findings to this study is not clear because all our sites are temporary and likely to be variable in the sense of O’Dwyer & Murphy (2021). More regular monitoring, both within and between filling cycles, of the salinity and other physicochemical parameters in our study sites is needed to understand whether some of these habitats are inherently more variable than others.
Between species variation
This study is one of few to experimentally compare the salinity tolerances of multiple closely related halophilic taxa not in the genus Artemia (Conte & Geddes, 1988; Browne & Wanigasekera, 2000; O'Dwyer & Murphy, 2021). The results suggest that, although all species demonstrated impressive euryhalinity, some Coxiella species have different upper salinity tolerances. According to the IC50 estimates, the upper salinity tolerances of Coxiella species rank from highest to lowest in the following order C. n. sp. 2, C. glauerti, C. pyrrhostoma, C. striatula, C. exposita and C. glabra. The difference between the most tolerant species (C. n. sp. 2, IC50 = 146.4 mS/cm) and the least tolerant species (C. glabra, IC50 = 94.1 mS/cm) was substantial. Statistical testing confirms that upper salinity tolerances in C. glauerti and C. n. sp. 2 were different (higher) from those of C. exposita and C. glabra. The upper salinity tolerances of C. striatula and C. pyrrhostoma were intermediate between these two groups but were not significantly different to each other or those of any other species. Obtaining data for additional populations of each species would improve the power of the statistical testing and might reveal further significant differences among species, although it could also just increase the amount intraspecific variation/overlap among species. Most tested species overlapped in their lower salinity limit, but some species seem to tolerate low salinity conditions better than others (e.g. C. glabra and C. glauerti compared with C. n. sp. 2).
In the experiments of Williams & Mellor (1991), individuals of C. striata from a single population in South Australia showed a broad salinity tolerance (lower IC50 = 3.8 mS/cm, upper IC50 = 125.3 mS/cm). Williams & Mellor (1991) also found that if C. striata individuals were directly transferred to tanks of higher or lower salinity without being allowed to gradually acclimatise their salinity tolerances lessened (lower IC50 = 10.4 mS/cm, upper IC50 = 111.7 mS/cm). These estimates suggest that the upper and lower limits of salinity tolerance in C. striata are broadly comparable to those obtained for the six Coxiella species in this study. However, we acknowledge that the C. striata results are not directly comparable with those obtained in the present study because of differences in experimental method. For example, Williams & Mellor (1991) raised and lowered salinities in their ‘gradual’ experiment every six days as opposed to every 24 h as done here.
Field vs experimental data
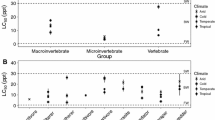
For each Coxiella species, the experimentally determined salinity tolerances were wider than the salinity range observed in field records. This suggests that the experimental conditions were conducive to activity over a broad range of salinities. The reason why individuals of Coxiella are active over a narrower range of salinities in the field could be because their field distributions are influenced by other variable/s in addition to salinity. In experiments testing the salinity tolerance of freshwater and halotolerant macroinvertebrates, artificially manufactured saline water was found to be less toxic to the macroinvertebrates than water from salt lakes at the same conductivity (Kefford, 2000). This suggests that electrical conductivity is not the only factor responsible for these species response to ‘salinity’ and that the increased toxicity of salt lake water may be due to the presence of elevated nutrients or other unknown pollutants (Kefford, 1998). It is also worth noting that the present study only tested salinity responses over a 24-h period and so the results do not necessarily suggest that Coxiella individuals could remain active at the same salinities for sustained time periods or could complete their life cycle at these salinities.
The discrepancy between experimentally determined and field records may also be explained if the current field records do not adequately cover the range of habitats/salinities occupied by the tested species. Since C. striatula, C. glabra, C. pyrrhostoma and to a lesser extent C. exposita have been sampled over their entire known distribution and from a range of different lakes (Lawrie et al., 2023), it is likely that field data for these species are a fair reflection of their salinity distributions. C. glauerti and C. n. sp. 2 are at the other extreme, having been encountered in relatively few lakes, which may explain the lack of field observations of both species at low salinities, despite the experimental data indicating that they can tolerate such conditions. Regardless, the field data included herein are the most comprehensive available for the studied species of Coxiella. It is worth noting that the identity of the species present at each site has been confirmed with genetic data (Lawrie et al., 2023). In contrast, Based on the results of Lawrie et al. (2023), the species-specific salinity data for Coxiella contained in some publications (e.g. Pinder et al. (2002), Pinder et al. (2005) and Timms (2009a)) are questionable due to uncertainties about the species identifications, although records for C. glabra and C. striatula in Halse (1981) and Edward (1983), respectively, are accurate.
Coxiella ecology
Despite overlap in their geographical distributions and salinity ranges, multiple Coxiella species rarely co-occur in the same lake. The potential influence of salinity on Coxiella species distributions is not clear. The experimental results and field records suggest that C. exposita and C. glabra have significantly less capacity to tolerate salinities as high as C. glauerti and C. n. sp. 2 and therefore these species could be excluded from some salt lakes occupied by C. glauerti and C. n. sp. 2 on the basis of salinity alone. However, the known geographic distributions of C. glabra and C. exposita do not overlap with those of C. glauerti and C. n. sp. 2 (Lawrie et al., 2023), making this situation unlikely. Their geographic distributions do overlap with those of C. pyrrhostoma and C. striatula and there is considerable overlap in their lower and upper IC50 tolerances but co-occurrence among any combination of these species is rare (Lawrie et al., 2023). It is suggested that, although the upper and lower limits of the salinity tolerance are broadly important in determining whether a Coxiella species can occur in a particular lake, within these limits other factors including food availability, hydroperiod, sediment characteristics, biological interactions and stochasticity are potentially at least as important (Williams et al., 1990; Kefford et al., 2004b; Timms, 2009b). More research is needed to understand the influence of biotic and abiotic processes on species occurrence in Coxiella and other invertebrate taxa in Australian salt lakes (Lawrie et al., 2021).
Two studied lakes, E22 and E23, are separated by a sandspit 30 m wide and yet support C. glauerti and C. n. sp. 2, respectively. These two lakes differ in their salinities with the more saline lake supporting the more saline tolerant species C. n. sp. 2 while C. glauerti occurs in E22. Each species is abundant in their respective lake but absent from the other despite the close proximity. The relatively low tolerance of C. glauerti to high salinities may explain why C. glauerti does not occur in E23 but not vice versa as the salinity of E22 is well within the tolerable salinity range of C. n. sp. 2. It is possible that Coxiella has colonised one or both lakes relatively recently and, given more time, the two species will disperse between them. However, this seems unlikely given that both E22 and E23 were previously sampled by Timms (2009a) (site numbers 33 and 34; note that Timms misidentified C. n. sp. 2 as C. glauerti), who also identified differences in the crustacean fauna of these two lakes suggesting that whatever factors are driving these differences they are applicable to a range of taxa.
Experimental design
The experiment used in this study measured salinity tolerance while holding constant some key abiotic variables, such as temperature and water pH/ionic composition. This is because we prioritised testing multiple populations and species over different combinations of abiotic variables. However, we recognise that salinity tolerance in other halophiles is influenced by the interactions between salinity and the ionic pH/composition of water (Bayly, 1969, 1972) and especially between salinity and temperature (Browne & Wanigasekera, 2000; Ismail et al., 2010). Nevertheless, although the exact tolerance estimates may have been different if different environment conditions had been used, the broader finding that Coxiella species have wide-ranging and high salinity tolerances is likely to be robust.
The experiment used Coxiella individuals that were recently collected from field sites with different environmental conditions, including salinity (see Table 2). Thus, there is the potential that the recent environmental history of the individuals has influenced the experimental results. This is a common problem in experimental studies (O’Dwyer & Murphy, 2021). For our experiment, there was no other option than to use individuals collected from the field as it is difficult and time-consuming to breed Coxiella individuals in the laboratory. To mitigate against this problem, snails were acclimatised to a constant set of environmental conditions in holding tanks in the laboratory for at least two days prior to being used in the experiment, noting that 24–28 h should be sufficient for body tissues to become isosmotic (Williams & Mellor, 1991). Also, individuals were only included in one experimental run and never reused. In addition, no relationship was found between the estimated salinity tolerance for a population and salinity of habitat from which the experimental individuals were collected except in the upper tolerance of C. exposita populations. Other factors that could not be controlled in the experiment were the age and size of snails, however, as much as possible the largest individuals available in each population were selected across runs to reduce the impact of these factors.
Conservation implications
This study has demonstrated that Coxiella species have very broad salinity tolerances. In this sense, relative to halotolerant taxa, Coxiella may be resilient to the drying/salinizing effects of climate change (Atkinson et al., 2021). Nevertheless, the experimental data only focussed on the tolerances of adults and it is unclear how increasing salinity might influence reproduction and recruitment as adults are generally more tolerant of osmotic stress than juveniles in other aquatic invertebrates (Kefford et al., 2004a). Also, although Coxiella species can tolerate high salinities, the experimental data demonstrated that there is an upper limit to these tolerances. This upper limit was higher for some species than others suggesting an uneven risk of extirpation within Coxiella. Field data suggest that the upper salinity limits of Coxiella species are noticeably less than those of some of the other halophilic invertebrates, such as Parartemia (Timms, 2014), Australocypris, Diacypris and Reticypris (Lawrie et al., 2021; Rahman et al., 2022), from Australian salt lakes. This is important to recognise as it suggests that the fate of salt lake invertebrates in response to salinisation should not be generalised at an ecosystem level as potential outcomes judged by the response of the most resistant halophilic fauna could grossly underestimate the implications for more sensitive taxa (Timms, 2005). Although Coxiella species may be able to aestivate during unfavourable periods, the maximum duration of aestivation is unknown and may also vary amongst species. Given that the regulatory mechanism for salinity tolerance could have arisen as an exaptation to drought conditions (Gomez-Mestre & Tejedo, 2005; Arribas et al., 2014), it would be interesting to know if salinity tolerance in Coxiella species is positively correlated with desiccation resistance.
Anecdotal evidence suggests that extirpations of Coxiella populations are happening in some locations, particularly in the wheatbelt region of Western Australia. For example, no live individuals were found in Lake Stubbs (Newdegate, Western Australia) in 2021 despite Williams & Mellor (1991) having previously collected Coxiella from this lake. Also, many salt lakes in the wheatbelt region have swathes Coxiella shells that appear to have formed recently (< 150 years), rather than being subfossils, but do not appear to contain any live individuals either in the water or aestivating (A. Lawrie unpublished data). These observations coincide with the progressive salinisation of lakes in this region and elsewhere since the onset of European land clearing, with many fresh or low salinity lakes now hypersaline (George et al., 2008). It is known that changes in hydrology of a water body can lead to the loss of a resident Coxiella population, i.e. C. striata from Lake Corangamite, Victoria (Williams, 1995) and a range of lakes on the Eyre Peninsula, South Australia (Timms, 2009b). Furthermore, extirpations in other aquatic invertebrates in the wheatbelt region have been linked to increasing salinisation of water bodies (Timms et al., 2009) and increasing aridity of climate (Atkinson et al., 2021). It seems reasonable to hypothesize that these two factors are causing the loss of Coxiella populations from salt lakes.
Conclusion
This study has experimentally demonstrated that six Coxiella species possess broad salinity tolerances that are amongst the highest recorded for gastropods from any environment. The experimental results revealed relatively little inter-population variation in upper salinity tolerance in most species but not all. Overall, there was little intra or inter-specific variation in lower salinity tolerance. The experimental results were consistent with field data in that the populations of species that are found in higher salinities in the field tended to show higher upper salinity tolerances in the experiments. However, for all but one species, the upper salinity tolerances observed in the experiment were higher than those observed in the field. This study demonstrates that, although Coxiella maybe more resilient to the effects of climate change relative to other halotolerant/freshwater taxa, some species will likely be at greater risk than others and extirpations have already occurred in south-western Australia. Future research needs to focus on how desiccation duration/tolerance varies between species and determining the sub-lethal effects of salinity on Coxiella.
Data availability
Data available upon request.
References
Andreeva, S., N. Andreev & R. Mikhaylov, 2020. Records of mollusks of the genus Caspiohydrobia Starobogatov 1970 (Gastropoda, Hydrobiidae) in salt rivers of the Caspian lowland. Biology Bulletin 47: 912–919.
Arribas, P., C. Andújar, P. Abellán, J. Velasco, A. Millán & I. Ribera, 2014. Tempo and mode of the multiple origins of salinity tolerance in a water beetle lineage. Molecular Ecology 23: 360–373. https://doi.org/10.1111/mec.12605.
Atkinson, S., D. Cale, A. Pinder, J. Chambers, S. Halse & B. J. Robson, 2021. Substantial long-term loss of alpha and gamma diversity of lake invertebrates in a landscape exposed to a drying climate. Global Change Biology 27: 6263–6279. https://doi.org/10.1111/gcb.15890.
Bayly, I. & W. D. Williams, 1966. Chemical and biological studies on some saline lakes of south-east Australia. Marine and Freshwater Research 17: 177–228.
Bayly, I., 1972. Salinity tolerance and osmotic behavior of animals in athalassic saline and marine hypersaline waters. Annual Review of Ecology and Systematics 3: 233–268.
Bayly, I. A., 1969. The occurrence of calanoid copepods in athalassic saline waters in relation to salinity and anionic proportions. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 17: 449–455.
Bozinovic, F., P. Calosi & J. I. Spicer, 2011. Physiological correlates of geographic range in animals. Annual Review of Ecology, Evolution, and Systematics 42: 155–179. https://doi.org/10.1146/annurev-ecolsys-102710-145055.
Bradley, T. J., 2009. Animal Osmoregulation, Oxford University Press, New York:
Brown, D. S., 1994. Freshwater Snails of Africa and Their Medical Importance, 2nd ed. Taylor and Francis, London.
Browne, R. & G. Wanigasekera, 2000. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. Journal of Experimental Marine Biology and Ecology 244: 29–44. https://doi.org/10.1016/S0022-0981(99)00125-2.
Cañedo-Argüelles, M., 2020. A review of recent advances and future challenges in freshwater salinization. Limnetica 39: 185–211. https://doi.org/10.23818/limn.39.13.
Carbonell, J., A. Millán & J. Velasco, 2012. Concordance between realised and fundamental niches in three Iberian Sigara species (Hemiptera: Corixidae) along a gradient of salinity and anionic composition. Freshwater Biology 57: 2580–2590. https://doi.org/10.1111/fwb.12029.
Céspedes, V., S. Pallarés, P. Arribas, A. Millán & J. Velasco, 2013. Water beetle tolerance to salinity and anionic composition and its relationship to habitat occupancy. Journal of Insect Physiology 59: 1076–1084. https://doi.org/10.1016/j.jinsphys.2013.08.006.
Conte, F. P. & M. C. Geddes, 1988. Acid brine shrimp: metabolic strategies in osmotic and ionic adaptation. Hydrobiologia 158: 191–200. https://doi.org/10.1007/Bf00026277.
Cox, J. C., 1868. A monograph of Australian land shells. W. Maddock, Sydney.
Croghan, P., 1958. The survival of Artemia salina (L.) in various media. Journal of Experimental Biology 35: 213–218.
Davis, G. M., 1981. Different modes of evolution and adaptive radiation in the Pomatiopsidae (Prosobranchia: Mesogastropoda). Malacologia 21: 209–262.
Deaton, L., 2009. Osmotic and ionic regulation in molluscs. In Evans, D. (ed), Osmotic and IONIC Regulation Cells and Animals CRC Press, Boca Raton: 107–133.
Devictor, V., J. Clavel, R. Julliard, S. Lavergne, D. Mouillot, W. Thuiller, P. Venail, S. Villeger & N. Mouquet, 2010. Defining and measuring ecological specialization. Journal of Applied Ecology 47: 15–25. https://doi.org/10.1111/j.1365-2664.2009.01744.x.
Dunn, O. J., 1964. Multiple comparisons using rank sums. Technometrics 6: 241–252.
Edward, D., 1983. Inland waters of Rottnest Island. Journal of the Royal Society of Western Australia 66: 41–47.
Ellis, P. & W. Williams, 1970. The biology of Haloniscus searlei Chilton, an oniscoid isopod living in Australian salt lakes. Marine and Freshwater Research 21: 51–70.
Filippov, A. & A. Y. Komendantov, 1996. The salinity tolerance of benthic invertebrates of the Aral Sea. International Journal of Salt Lake Research 4: 251–263.
Filippov, A. & F. Riedel, 2009. The late Holocene mollusc fauna of the Aral Sea and its biogeographical and ecological interpretation. Limnologica 39: 67–85. https://doi.org/10.1016/j.limno.2008.04.003.
Gaston, K. J., 2003. The Structure and Dynamics of Geographic Ranges, Oxford University Press
Geddes, M., 1981. The brine shrimps Artemia and Parartemia: comparative physiology and distribution in Australia. Hydrobiologia 81: 169–180. https://doi.org/10.1007/Bf00048714.
Geddes, M., P. De Deckker, W. D. Williams, D. Morton & M. Topping, 1981. On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 81: 201–222. https://doi.org/10.1007/Bf00048717.
George, R., J. Clarke & P. English, 2008. Modern and palaeogeographic trends in the salinisation of the Western Australian Wheatbelt: a review. Soil Research 46: 751–767. https://doi.org/10.1071/Sr08066.
Gerhard, D. & C. Ritz, 2015. medrc: Mixed Effect Dose-Response Curves R package version 00–73.
Gomez-Mestre, I. & M. Tejedo, 2005. Adaptation or exaptation? an experimental test of hypotheses on the origin of salinity tolerance in Bufo calamita. Journal of Evolutionary Biology 18: 847–855. https://doi.org/10.1111/j.1420-9101.2004.00878.x.
Halse, S., 1981. Faunal assemblages of some saline lakes near Marchagee, Western Australia. Marine and Freshwater Research 32: 133–142. https://doi.org/10.1071/Mf9810133.
Hammer, U. T., 1986. Saline lake ecosystems of the world, W. Junk, Dordrecht
Hochberg, Y., 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800–802.
Hollander, M., D. A. Wolfe & E. Chicken, 1973. Nonparametric Statistical Methods, John Wiley, Hoboken
Iredale, T., 1943. A basic list of the fresh water mollusca of Australia. The Australian Zoologist 10: 188–230.
Ismail, H. N., J. G. Qin & L. Seuront (eds), 2010. Thermal and Halo Tolerance of a Brackish Cladoceran Daphniopsis australis (Sergeev & Williams). Nova Science Publisher, New York.
Kameda, Y. & M. Kato, 2011. Terrestrial invasion of pomatiopsid gastropods in the heavy-snow region of the Japanese Archipelago. BMC Evolutionary Biology 11: 1–14. https://doi.org/10.1186/1471-2148-11-118.
Kefford, B. J., 1998. Is salinity the only water quality parameter affected when saline water is disposed in rivers? International Journal of Salt Lake Research 7: 285–300.
Kefford, B. J., 2000. The effect of saline water disposal: implications for monitoring programs and management. Environmental Monitoring and Assessment 63: 313–327. https://doi.org/10.1023/A:1006201512469.
Kefford, B. J., A. Dalton, C. G. Palmer & D. Nugegoda, 2004a. The salinity tolerance of eggs and hatchlings of selected aquatic macroinvertebrates in south-east Australia and South Africa. Hydrobiologia 517: 179–192. https://doi.org/10.1023/B:HYDR.0000027346.06304.bc.
Kefford, B. J., P. J. Papas, L. Metzeling & D. Nugegoda, 2004b. Do laboratory salinity tolerances of freshwater animals correspond with their field salinity? Environmental Pollution 129: 355–362.
Kefford, B. J., G. L. Hickey, A. Gasith, E. Ben-David, J. E. Dunlop, C. G. Palmer, K. Allan, S. C. Choy & C. Piscart, 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: Eastern Australia, France. Israel and South Africa. Plos One 7: e35224.
Kosicka, E., A. Lesicki & J. R. Pieńkowska, 2020. Molluscan aquaporins: an overview, with some notes on their role in the entry into aestivation in gastropods. Molluscan Research 40: 101–111. https://doi.org/10.1080/13235818.2020.1716442.
Lawrie, A. D. A., J. Chaplin & A. Pinder, 2021. Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes. Marine and Freshwater Research 72: 1553–1576. https://doi.org/10.1071/MF21088.
Lawrie, A. D. A., J. Chaplin, L. Kirkendale, C. Whisson, A. Pinder & M. C. Mlambo, 2023. Phylogenetic assessment of the halophilic Australian gastropod Coxiella and South African Tomichia resolves taxonomic uncertainties, uncovers new species and supports a Gondwanan link. Molecular Phylogenetics and Evolution 184: 107810. https://doi.org/10.1016/j.ympev.2023.107810.
McEvoy, P. & P. Goonan, 2003. Salinity is not necessarily bad for biodiversity: case studies of invertebrates from South Australian streams and River Murray wetlands. Records of the South Australian Museum Monograph Series 7: 131–134.
Menke, K., 1843. Molluscorum Novae Hollandiae Specimen. Libraria Aulica Hahniana: Hannover, Germany.
Morelet, A., 1889. Coquilles nouvelles de l’Afrique méridionale. Journal de Conchyliologie 37: 5–20.
O’Dwyer, J. E. & N. P. Murphy, 2021. Long term environmental stability drives reduced stress tolerance in salt lake invertebrates. Rethinking Ecology 6: 49–64.
Pinder, A. M., S. A. Halse, R. J. Shiel, D. J. Cale & J. M. McRae, 2002. Halophile aquatic invertebrates in the wheatbelt region of south-western Australia. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 28: 1687–1694.
Pinder, A. M., S. A. Halse, J. M. McRae & R. J. Shiel, 2005. Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543: 1–24. https://doi.org/10.1007/s10750-004-5712-3.
Pinheiro, J., D. Bates, S. DebRoy & Sarkar D, 2015. nlme: linear and nonlinear mixed-effects models. R package version 3.1–103. R package version 3.1–130.
Rahman, M., J. Chaplin & A. Pinder, 2022. The biology of giant ostracods (Crustacea, Cyprididae), a review focusing on the Mytilocypridinae from Australian inland waters. Marine and Freshwater Research 74: 1–19. https://doi.org/10.1071/Mf22092.
Reeve, L. A., 1842. Conchologia Systematica, or complete system of conchology. In Which the Lepades and Conchiferous Mollusca are Described and Classified According to their Natural Organization and Habits. Longman, Brown, Green, & Longman’s, London.
Reid, R., A. Oehlert, E. Suosaari, C. Demergasso, G. Chong, L. Escudero, A. Piggot, I. Lascu & A. Palma, 2021. Electrical conductivity as a driver of biological and geological spatial heterogeneity in the Puquios, Salar de Llamara, Atacama Desert, Chile. Scientific Reports 11: 1–18. https://doi.org/10.1038/s41598-021-92105-2.
Ritz, C., F. Baty, J. C. Streibig & D. Gerhard, 2015. Dose-response analysis using R. PLoS One 10: e0146021. https://doi.org/10.1371/journal.pone.0146021.
Saccò, M., N. E. White, C. Harrod, G. Salazar, P. Aguilar, C. F. Cubillos, K. Meredith, B. K. Baxter, A. Oren & E. Anufriieva, 2021. Salt to conserve: a review on the ecology and preservation of hypersaline ecosystems. Biological Reviews 96: 2828–2850. https://doi.org/10.1111/brv.12780.
Salvador, R. B., F. S. Silva & M. E. Bichuette, 2022. Phylogenetic position of the relict South American genus Idiopyrgus Pilsbry, 1911 (Gastropoda, Truncatelloidea), with the description of two new cave species. Zoosystematics and Evolution 98: 365–375. https://doi.org/10.3897/zse.98.90797.
Timms, B. V., 1983. A study of benthic communities in some shallow saline lakes of western Victoria, Australia. Hydrobiologia 105: 165–177. https://doi.org/10.1007/Bf00025186.
Timms, B. V., 2005. Salt lakes in Australia: present problems and prognosis for the future. Hydrobiologia 552: 1–15. https://doi.org/10.1007/s10750-005-1501-x.
Timms, B. V., 2009a. Study of the saline lakes of the Esperance Hinterland, Western Australia, with special reference to the roles of acidity and episodicity. Natural Resources and Environmental Issues 15: 215–224.
Timms, B. V., 2009b. A study of the salt lakes and salt springs of Eyre Peninsula, South Australia. Hydrobiologia 626: 41–51. https://doi.org/10.1007/s10750-009-9736-6.
Timms, B. V., 2014. A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca). Journal of Biological Research (thessalon) 21: 21. https://doi.org/10.1186/2241-5793-21-21.
Timms, B. V., P. Coleman & J. Cooper, 2014. Seagull Lake, Western Eyre Peninsula, South Australia: A Saline Lake to Benefit from Climate Change? Transactions of the Royal Society of South Australia 138: 161–180.
Timms, B. V., A. M. Pinder & V. S. Campagna, 2009. The biogeography and conservation status of the Australian endemic brine shrimp Parartemia (Crustacea, Anostraca, Parartemiidae). Conservation Science Western Australia 7: 413–427.
Williams, W. D., 1964. A contribution to lake typology in Victoria, Australia. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie: Verhandlungen 15: 158–168.
Williams, W. D., 1995. Lake Corangamite, Australia, a permanent saline lake: conservation and management issues. Lakes & Reservoirs: Research & Management 1: 55–64.
Williams, W. D., 1998. Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381: 191–201. https://doi.org/10.1023/A:1003287826503.
Williams, W. D., A. Boulton & R. Taaffe, 1990. Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197: 257–266. https://doi.org/10.1007/Bf00026955.
Williams, W. D. & M. W. Mellor, 1991. Ecology of Coxiella (Mollusca, Gastropoda, Prosobranchia), a snail endemic to Australian salt lakes. Palaeogeography, Palaeoclimatology, Palaeoecology 84: 339–355. https://doi.org/10.1016/0031-0182(91)90053-T.
Acknowledgements
Samples for this project were collected under licences from the Department of Biodiversity, Conservation and Attractions and the Department of Primary Industries and Regional Development. The authors thank Christian Ritz for his advice regarding the medrm R package. Angus Lawrie was supported by a Commonwealth Supported Research Training Program while conducting this research. Thank you to the two anonymous reviewers whose feedback greatly improved this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding was supported by Australian Government Research Training Program Scholarship, Research Training Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.






Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lawrie, A.D., Chaplin, J., Rahman, M. et al. Experimental and field evidence suggests extreme salinity tolerances in Coxiella gastropods from Australian salt lakes. Hydrobiologia 851, 205–221 (2024). https://doi.org/10.1007/s10750-023-05329-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05329-w