Abstract
Global decline of freshwater mussels (Unionoida) is threatening biodiversity and the essential ecosystem services that mussels provide. As filter-feeding organisms, freshwater mussels remove phytoplankton and suspended particles from the water. By filtering bacteria, freshwater mussels also decrease pathogen loads in the water. The objective of this study was to evaluate whether the common freshwater bivalve Anodonta anatina (duck mussel) could remove the bacterial fish pathogen Flavobacterium columnare from the water. Mussels reduced bacteria in both of the two experiments performed, so that the bacterial concentration at the end of the 96-h monitoring in mussel treatments was only 0.3–0.5 times that of the controls. Surprisingly, mussels did not reduce algal cell concentration statistically significantly. Mussel behavior (shell openness, foot position, and movement) was not affected by the presence of bacteria or algae, except for biodeposition formation, which was greatest in algal-fed treatments, followed by bacterial-fed treatments and controls, respectively. The intestines of bacteria-incubated A. anatina harbored F. columnare, suggesting that mussels ingested the bacteria. Present results suggest that freshwater mussels may also have a potential to mitigate aquaculture pathogen problems, as well as play a role in water quality management.
Similar content being viewed by others
Introduction
Freshwater mussels (Unionoida) are one of the most threatened animal group in the world (Lydeard et al., 2004; Lopes-Lima et al., 2017). In the recent decades, a number of freshwater mussel species have gone extinct (Haag & Williams, 2014). Mussel populations are declining due to pollution, habitat degradation and fragmentation, introduced species, and the loss of obligatory fish hosts (Bogan, 2008; Geist, 2010; Ferreira-Rodríguez et al., 2019), reducing the biological diversity in lakes and rivers. Freshwater mussels carry out important ecosystem services via their filtration and burrowing activity, including nutrient recycling and the deposition of organic matter from the water column to the bottom in the form of feces and pseudofeces (e.g., Vaughn, 2018). The biodeposition of feces and pseudofeces (the non-ingested particles loosely wrapped in mucus and ejected without undergoing the digestive process; Berg et al., 1996) by freshwater mussels provides a nutrient-rich and easily assimilated food source for benthic microbes and invertebrates, which supports fish populations and links multiple trophic levels in aquatic ecosystems (Howard & Cuffey, 2006; Vaughn et al., 2008; Vaughn, 2018). Therefore, in addition to biodiversity reduction per se, the decline of freshwater mussels can have a marked effect on the ecosystem.
Bivalves’ potential role in water quality management, their “bivalve environmental services”, has already been recognized in marine systems (e.g., nutrient trapping connected with mussel production) (Nielsen et al., 2016; Clements & Comeau, 2019; Taylor et al., 2019; Kotta et al., 2020). Mussels’ effectiveness in nutrient and particle removal and in wastewater treatment has also been shown in freshwater systems (Strayer, 2014; Lummer et al., 2016; Mezzanotte et al., 2016; Hoellein et al., 2017; Kreeger et al., 2018). Thus, freshwater mussels can potentially be utilized in water quality management to reduce nutrient effluents and eutrophication (Kreeger et al., 2018). Depending on their ability to remove bacteria, freshwater mussels could also be used to reduce disease outbreaks. Several researchers have found that the dreissenid mussel Dreissena polymorpha (Pallas, 1771) can clear the pathogenic bacterium Escherichia coli (Migula, 1895) Castellani & Chalmers, 1919 from water in laboratory experiments (Silverman et al., 1995), in field conditions (Cotner et al., 1995), and from treated effluents of municipal waste water (Mezzanotte et al., 2016). Unionidae freshwater mussels are also capable of filtering bacteria (Jørgensen et al., 1984; Kryger & Riisgård, 1988; Nichols & Garling, 2002; Christian et al., 2004Vanderploeg et al., 2011b), including E. coli (Silverman et al., 1995; Ismail et al., 2015). However, the ability of freshwater mussels to eliminate bacterial fish pathogens has not been investigated.
The aim of the present study was to investigate the capability of the duck mussel, Anodonta anatina (Linnaeus, 1758), to remove the bacterium Flavobacterium columnare Bernardet & Grimont 1989 from the water column in laboratory conditions. Anodonta anatina is a unionid mussel that commonly occurs in the lakes and rivers of Europe (Lopes-Lima et al., 2016). This mussel has been shown to filter Diplostomum pseudospathaceum Niewiadomska, 1984 larvae from water, thereby reducing the transmission of this trematode parasite from aquatic snails to its fish host (Gopko et al., 2017). More importantly, A. anatina has the potential to filter and ingest colonial and filamentous cyanobacteria (Bontes et al., 2007). Flavobacterium columnare is a freshwater bacterium and an opportunistic fish pathogen that causes columnaris disease (“warm water disease”) in fish. Flavobacterium columnare can infect a range of fish species throughout the world, such as salmon, trout, rainbow trout, channel catfish, carp, perch, pike, eel, and tilapia (Declercq et al., 2013). Columnaris disease is one of the most harmful diseases in channel catfish, Ictalurus punctatus (Rafinesque, 1818) farming in the USA, leading to approximately $30 million yearly losses (Wagner et al., 2006). It is also a very difficult problem in young salmonid culture, causing high mortality and the use of antibiotics (Pulkkinen et al., 2010). This pathogen can also be found in natural lakes and rivers (Kunttu et al., 2012) and has been isolated from tissues of the unionid mussel Villosa iris (I.Lea, 1829) (Clinch River, USA; Starliper et al., 2008).
Our hypothesis was that A. anatina would be able to remove F. columnare from the water, manifested as a lower average bacterial concentration in the water with mussel treatment as compared to the control water without mussel. As a methodological control for the feeding of mussels, we established a treatment group fed with a commercial microalgae product commonly used for mussel feeding. As the bacterial suspension may affect the behavior of mussels, especially the filtration activity, monitoring of mussel behavior was included to the study. In addition, as the biodeposit formation can indicate filtration of matter from water, biodeposition formation was also monitored during the experiment. The present study is the first effort to evaluate the efficiency of freshwater mussels to remove the harmful bacterial fish pathogen from the water.
Materials and methods
Mussel collection and preparation
In total, 30 A. anatina individuals (Table 1) were collected from Lake Koijärvi, Finland (60.97°N, 23.73°E) during September 2016. The mussels were transported to Konnevesi Research Station, University of Jyväskylä and were kept in a 163 L flow-through tank with sand on the bottom until the experiments began. The mussels were not fed, but they received food from incoming Lake Konnevesi water during the maintenance period. In January 2017, the mussels were transported to the laboratory of the Department of Biological and Environmental Science, University of Jyväskylä. The mussels were placed in a large bucket of well water, aerated with an aquarium air pump, and kept on a 12 h light–dark cycle at ~ 17 °C. In the laboratory during the pre-experiment maintenance, the mussels were fed ad libitum with a commercial phytoplankton product (Shellfish Diet 1800®, SFD, Reed Mariculture, USA) containing six marine microalgae: Isochrysis sp., Pavlova sp., Tetraselmis sp., Chaetoceros calcitrans (Paulsen) H.Takano, Thalassiosira weissflogii (Grunow) G.A.Fryxell & Hasle, and Thalassiosira pseudonana Hasle & Heimdal. The mussels were kept without food for 24 h before starting the experiments until they no longer released excrement. The water temperature at Konnevesi Research Station during the maintenance period was initially 17 °C in September; it gradually declined to 3 °C in December 2016, but it was artificially gradually increased to 17 °C in the last two weeks before the experiments, which were conducted at 17 °C.
Preparation of bacterial suspension, Shieh medium and agar plates
The F. columnare strain (B613) used in the experiments was isolated from a water sample taken from the shore of Lake Kynsivesi (62°25″N, 26°15″E) in 2014; it was then cultivated in a modified Shieh medium (Song et al. 1988) and stored at -80 °C with 10% glycerol and 10% fetal calf serum. In order to prepare the bacterial solution for the experiments, 20 μl from the frozen stock was added to 5 ml of modified Shieh medium and incubated with continuous stirring at 120 rpm for 24 h at room temperature. One ml of the overnight-grown bacterial suspension was further added to 9 ml of modified Shieh medium, with another incubation at room temperature overnight. The optical density (OD) of the bacterial suspension was measured with a spectrophotometer at 595 nm and converted to a bacterial concentration using a pre-established relationship between OD and colony-forming units (CFUs); this was done to allow the researchers to adjust the initial bacteria concentration levels during the experiments.
Modified Shieh medium was prepared according to Song et al. (1988) to serve as a selective growth medium to isolate F. columnare. Agar plates were used to plate-count the viable bacterial concentrations; here, 10 g of agar powder were added per liter and supplemented with tobramycin in order to inhibit the growth of bacteria other than F. columnare (Decostere et al., 1997).
Experiment 1
The first experiment to evaluate A. anatina’s ability to remove F. columnare from water began in February 2017 and included 5 treatments with 5 replicates each (replicate = 1 sediment/sand-free aquarium filled with 5 L of well water with or without a mussel individual; see below). A schematic presentation of the experimental design is given in the Supplementary Fig. 1. The aquaria were aerated and kept on a light–dark cycle of 12 h:12 h at ~ 17 °C. The experimental treatments were as follows:
-
(1)
Bacteria and mussel: A known concentration (5 × 105 CFU ml−1) of F. columnare with an individual mussel to evaluate the potential of A. anatina to filter out F. columnare.
-
(2)
Bacteria control: A known concentration (5 × 105 CFU ml−1) of F. columnare without a mussel was used to study changes in the bacterial concentration of the water in the absence of the mussel.
The null hypothesis was that there was no difference in bacterial concentration between treatments (1) and (2).
-
(3)
Algae and mussel: A known concentration of algae with a mussel; this served as a reference treatment to study A. anatina’s algal filtration.
-
(4)
Algae control: A known concentration of algae without a mussel was used to track changes in algae concentration in the water in the absence of the mussel.
In treatments (3) and (4), 35 ml of Shellfish Diet 1800® was added to 1.1 L water; then, 100 ml of this suspension was added to each aquarium.
The null hypothesis was that there was no difference in algae concentration between treatments (3) and (4).
-
(5)
Mussel control: A mussel without F. columnare and without algae was used to study mussel behavior without the presence of bacteria and algae.
The null hypothesis was that there was no difference in behaviors between treatments (1), (3), and (5).
Experiment 2
The second experiment began in March 2017 to verify the results of Experiment 1 in regard to bacterial filtration; it used a bacterial inoculation that was an order of magnitude lower than in Experiment 1 (see the Results section and Fig. 1). A schematic presentation of the experimental design is given in the Supplementary Fig. 2. The aquaria were aerated and kept on a light–dark cycle of 12 h:12 h at ~ 17 °C. The experimental treatments were as follows:
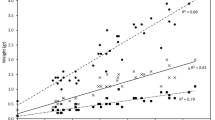
Bacterial concentration (CFU ml−1) (A) in aquaria with mussels and without mussels at different time points (N = 5) (mean ± SD) in the 1st experiment, in the 2nd experiment (B), and algal cell concentration (cell ml−1) (C) in aquaria with mussels and without mussels at different time points (N = 5) (mean ± SD)
(1) Bacteria and mussel: A known concentration of F. columnare with an individual mussel to evaluate A. anatina’s potential to filter F. columnare; 9 replicate aquaria with a single mussel in each were used.
(2) Bacteria control: A known concentration of F. columnare without a mussel was used to study changes in bacterial concentration in the absence of the mussel; 5 replicate aquaria were used.
The null hypothesis was that there was no difference in bacterial concentration between treatments (1) and (2).
Bacterial sampling from the water
Water samples were taken from all the aquaria prior to starting each experiment to exclude the possibility of prior contamination by F. columnare. The concentration of bacteria was evaluated from 100 μl water samples from treatments with added bacteria (bacteria and mussel, bacteria control) at time points 0 h, 6 h, and 24 h; 1 sample per aquarium was evaluated. In order to decrease the observational error associated with reducing bacterial levels at time points 48 h and 96 h, the number of repeated water samples taken per aquarium was increased to three. In Experiment 2, 100 μl of water was taken at time points 0 h, 48 h, and 96 h; 2 repeated samples were taken per aquaria. A dilution series (10−1–10−7) of each water sample was prepared and cultured on Shieh agar plates; colony formation was checked after 48 h. At the end of both experiments, treatments without bacteria were checked as described above (2 × 100 μl water samples per aquarium) to exclude potential contamination from added bacteria during the experiment.
Bacterial sampling from intestines and gonads
To examine if mussels can ingest bacteria and become infected with F. columnare, bacterial samples were taken from intestine and gonad of the mussels from all treatments at the end of both experiments. To avoid bacterial contamination from the aquarium water, mussel shell surfaces were sprayed with 70% ethanol before dissection. After opening the valves, the mussel body was cut in 2 so that a cross-section through the gonads—and the intestine winding through the gonads—could be accessed. Then, the bacterial samples were collected with a sterile loop and cultured on Shieh agar plates supplemented with tobramycin. In Experiment 1, bacterial sampling was done first from the intestine and then from the gonads. In Experiment 2, the sampling order was reversed to minimize possible contamination of the gonads from the intestine.
Algal cell sampling
To investigate A. anatina’s filtration capability, algal cell concentration was monitored from 3 replicate water samples per aquarium at different time points (0 h, 6 h, 24 h, 48 h) using a microscope counting chamber (Bürker, Marienfeld, Germany, depth = 0.1 mm, square width = 0.25 mm). After 24 h, algal cell concentration declined sharply in both treatments, and monitoring was discontinued.
Mussel behavior
In order to check the possible effect of the bacterial and algal diets on the mussels’ behavior (for example, a complete inactivation of mussels when incubated with bacteria), in both experiments, A. anatina behavior was recorded 3 times per day at 9:00, 12:00, and 15:00 h in a 2 min visual inspection for 99 h. The traits that were monitored included shell openness, foot position (extension or contraction), movement, and biodeposition (feces and pseudofeces) formation since the previous monitoring. Shell openness was scored as follows: fully closed (siphons completely invisible) = 0, slightly opened (siphons are still invisible) = 1, half open (siphons visible, partly protruding) = 2, fully open (siphons fully visible, fully protruding) = 3. Foot position was graded as follows: foot in = 0, foot partly out = 1, foot out = 2. Movement (position changes in the mussel) was monitored by comparing mussel position to a mark on the lid of each aquarium denoting the position at the previous inspection. It was scored as follows: movement = 1, no movement = 0. Biodeposition presence was classified into 2 categories: without biodeposition = 0, and with biodeposition = 1.
Trematode parasitism, sex, and reproduction of mussels
Possible trematode parasitism (parasite species, intensity of infection) in the mussels was examined at the end of each experiment when the bacterial sampling from the intestine and gonads was performed; this was done because trematodes can have a marked influence on mussel physiology (Taskinen, 1998; Jokela et al., 2005). The gonads are the main site of trematode infection in A. anatina (Taskinen et al., 1997). Therefore, a 2–3 mm slice across the gonads was dissected after the bacterial sampling was conducted, pressed between large glass plates, and examined microscopically for trematode sporocysts using transmitted light. This was accompanied with the sex determination of the mussel and an estimation of the intensity of the infection (Taskinen et al., 1994; Taskinen & Valtonen, 1995). The intensity of the trematode infection was scored as follows: uninfected = 0, light infection (one sporocyst tubule or one group of sporocyst tubules) = 1, moderate infection from > 1 group of sporocyst tubules to almost complete replacement of mussel gonad tissue by sporocysts = 2, and heavy infection (gonad tissue completely replaced by trematode sporocysts) = 3 (Taskinen et al., 1994).
Statistical analyses
The statistical analyses of bacterial and algal concentrations were performed using IBM SPSS software (version 24.0.0). Cell concentrations (the CFUs of F. columnare and the counts of algal cells) were used as response variables; aquaria were used as the statistical units. General Linear Model (GLM) repeated measures analyses were performed to compare differences in bacterial and algal concentration in time (the within-subject factor) between treatments with and without a mussel (the between-subject factor). The differences between different time points were further examined using within-subject contrast (“simple”). Levene’s test was used to assess the homogeneity of variance, and the Greenhouse–Geisser correction was applied to correct the number of degrees of freedom when the sphericity assumption was not valid. Differences in mussel behavior (shell openness, foot position, movement, biodeposition formation) at different time points between the treatments (mussel only, mussel + bacteria, mussel + algae) were analyzed using mixed effects logistic regression (GLMM) in R (version 4.0.2.) statistics software and Lme4 package (R Development Core Team, 2015). The interdependence of repeated observations from the same individual mussel during the experiment was accounted for by treating mussel identity as a random variable. To be able to fit the models using binomial (logit) distribution, all behavioral data were recoded as 0 or 1 before the analysis. Thus, shell openness was recategorized as 0 (valves fully closed) and 1 (valves slightly, half or fully open), and foot position as 0 (foot in) and 1 (foot partly or completely out).
The differences in the intensity of trematode infection between treatment groups in Experiment 1 scored from zero (uninfected) to 3 (heavily infected) could not be tested with a Chi-square test because expected values were less than 5. Instead, we used Fisher´s exact test extended to 3 × 3 contingency table (McDonald , 2014). In addition, the difference in the mean intensity of the trematode infection was tested between the “bacteria and mussel” treatments in both experiments with a t-test. Differences between treatments were considered significant if p < 0.05.
Results
Effect of mussel filtration on bacterial concentration (Experiment 1)
At the beginning of Experiment 1, the mean concentration of bacteria was similar in both treatment groups with F. columnare (Fig. 1A). During the next 48 h, the bacterial concentration increased in both treatments, but the increase was more pronounced in the treatment without a mussel than in the treatment with a mussel. Between 48 and 96 h, the bacterial concentration decreased in both treatments (Fig. 1A). The bacterial concentration was lower in the treatment with a mussel than in the treatment without a mussel (Table 2, between-subject effects, F1,8 = 16.31, p = 0.004). However, this difference became significant only after 96 h of the experiment, as indicated by the significant interaction between time and treatment and the contrasts between the levels of the within-subject factor and treatment (time x treatment) (Table 2). Thus, it took 48 h from the beginning of the experiment before the removal of the bacteria by the mussels was apparent (Fig. 1A). Thereafter, the mussels reduced the bacteria so that the bacterial concentration in mussel treatment was 0.7–0.5 times that of the controls at 48 and 96 h, respectively (Fig. 1A). Thus, the mussels removed half the bacteria within 96 h as compared to the controls.
Effect of Mussel Filtration on Bacterial Concentration (Experiment 2)
In Experiment 2, a similar pattern in bacterial concentrations to those in Experiment 1 was observed. The bacterial concentrations were similar in the beginning of the experiment in the treatments with and without a mussel, respectively (Fig. 1B). At 48 h, the mean concentration of F. columnare had increased in both treatments, but with a mussel the increase was approximately half of that in the treatment without a mussel. Between 48 and 96 h, the bacterial concentrations decreased in both treatments (Fig. 1B). The bacterial concentration was lower in the treatment with a mussel than in the treatment without a mussel (Table 2, between-subject effects, F1,12 = 18.55, p = 0.001). Contrasts between the levels of the within-subject factor and treatment (time x treatment) indicated that this difference was significant after 48 h but not after 96 h of beginning the experiment (Table 2). Mussels reduced the bacterial concentration in mussel treatment to 0.5 and 0.3 times that of the controls at 48 and 96 h, respectively (Fig. 1B). Thus, the mussels removed two-thirds of the bacteria within 96 h as compared to the controls.
Gonad and Intestine Analysis (Experiments 1 and 2)
In Experiment 1, F. columnare was isolated both from the mussels’ intestines and gonads in the treatment with added bacteria, but not in the other treatment groups (i.e., the mussels with algae and the mussels kept in clean water). At the individual (mussel) aquarium level, among the 5 replicates of mussel + bacterium treatment, 3 mussels had F. columnare in both the intestine and the gonads, 1mussel had F. columnare in the intestine but not in the gonads, and 1 tested negative (no bacterium in either the intestine or in the gonads). In Experiment 2, among the 9 replicates of mussel and bacteria, F. columnare was only found in the intestines of 8 individuals; it was not found in the gonads.
Effect of Mussel Filtration on Algal Cell Concentration (Experiment 1)
The mean concentration of algal cells (Shellfish Diet) decreased between the start of the experiment and 48 h in both treatments with and without a mussel (Fig. 1C, Table 3, within-subject effect [time], F3,24 = 83.20, p = 0.001). However, the presence of A. anatina did not affect algal cell concentration (Table 3, F1,8 = 1.872, p = 0.208).
Mussel Behavior
Shell openness (Experiments 1 and 2)
In both experiments, the mussels in all treatments had open or partly open valves at the beginning of the experiment. The mussels gradually closed their valves during the first 24 h but started opening their shells again toward the end of the experiment (Fig. 2A and E). In Experiment 1, the presence of bacteria did not affect the mussels’ shell openness as compared to the mussels with algal cells or those in clean water (GLMM, p > 0.05, Table 4).
Shell openness (A), foot position (B), mussel movement (C) and biodeposition formation (D) in different treatments with mussels, added bacteria and algae at different time points (mean ± SD) in the 1st experiment. Shell openness (E), foot position (F), mussel movement (G) and biodeposition formation (H) in treatment with mussels and bacteria at different time points (mean ± SD) in the 2nd experiment
Foot position (Experiments 1 and 2)
Foot position scores revealed that the mussels kept their feet substantially contracted (Fig. 2B and F). In Experiment 1, the bacterial presence did not affect the foot position as compared to the mussels exposed to the algae or those kept in clean water (GLMM p > 0.05, Table 4).
Movement (Experiments 1 and 2)
Anodonta anatina in all treatment groups in both experiments exhibited a continuous and coherent pattern in movement behavior throughout the experiment (Fig. 2C and G). The presence of bacteria had a slight effect in decreasing mussels’ movement activity (GLMM, the relative change in comparison to mussel only treatment 0.69 ± 0.36, p = 0.053, Table 4), while the movement activity of mussels with algae was lower than mussels in clean water (GLMM, the relative change in comparison to mussel only treatment 0.75 ± 0.36, p = 0.036, Table 4).
Biodeposition (Experiments 1 and 2)
In Experiment 1, there were statistically significant differences between the bacterial, algal, and clean water treatments in terms of mean biodeposition formation (GLMM, the relative change for mussel + bacteria treatment 1.37 ± 0.49, p = 0.006 and for mussel + algae treatment 5.93 ± 1.10, p < 0.001 in comparison to mussel only treatment, Table 4); thus, biodeposition formation was highest with the algal diet, second highest with the bacterial diet, and lowest with clean water (Fig. 2D). Biodeposits were found at time point 24 h in all treatment groups (Fig. 2D). After this time as well as toward the end of the experiment, biodeposition formation almost ceased in the group kept in clean water. In the treatment with added bacteria, biodeposition formation showed temporary cessation after 24 h, but it started again by 54 h and continued until the end of the experiment. In the treatment with algae, biodeposition formation remained high and constant until the end of the experiment (Fig. 2D). In Experiment 2, biodeposition formation largely resembled the pattern observed in the corresponding treatment (bacteria) of Experiment 1, with an increasing trend from 30 h onward (Fig. 2H).
Trematode Parasitism and Sex
Almost all the mussels in both experiments (14 out of 15 and 7 out of 9 in Experiments 1 and 2, respectively) were infected with the trematode parasite Rhipidocotyle fennica Gibson et al., 1992 (Table 1). One individual in Experiment 2 was also infected with the trematode Cercaria duplicata von Baer, 1827 and with R. fennica (a double infection). There were no statistically significant differences in the intensity of trematode infection between the mussels in the bacterial, algal, and clean water treatments in Experiment 1 (Fisher´s exact test p = 0.829), nor were there differences between the mussels in the bacterial treatment in Experiments 1 and 2 (t = 0.350, p = 0.732). Because of the sterilizing trematode infection in the gonads (Taskinen & Valtonen, 1995), the sex of the mussels could not be determined for most of the individuals (Table 1).
DISCUSSION
As hypothesized, the presence of A. anatina reduced the mean concentration of the freshwater fish pathogen F. columnare in the water column so that at its lowest, only one-third of the concentration of the control level was observed in the mussel aquaria. Detecting F. columnare in the intestines of the mussels exposed to the bacteria, and formation of biodeposition in the presence of bacteria, suggests that the decrease in bacterial concentration was due to mussel filtration. In addition, other possible factors that could have caused or contributed to the reduction in concentration of F. columnare are, for example, competitive interactions between F. columnare and the microbiota present in and on the mussels (Rubiolo et al. 2019) or antimicrobial properties in mussel mucus (Cilia & Fratini 2018).
Filtration capacity in bivalves varies based on particle size and quality, among other things (Faust et al., 2009; Tuttle-Raycraft & Ackerman, 2018). Freshwater mussels can capture a wide range of particles sized 1–40 μm, including bacteria—at least large bacteria (Strayer, 2008). Flavobacterium columnare is a rod-shaped bacterium measuring 4–10 μm in length and 0.3–0.5 μm in width (Declercq et al., 2013). Thus, by its size, F. columnare should be suitable for filtering by freshwater mussels, as suggested by the current results.
Isolation of F. columnare only from the aquaria with added bacterium indicates that the bacterium was not present in the experimental mussels originating from the lake, or in the borehole water source used in the study, and that there was no cross-contamination between aquaria from adding the bacteria. In Experiment 1, a difference in bacterial concentration between the mussel treatment and control was not observed until 24–48 h. In addition, significant biodeposition production in the bacterial diet treatment also started late in Experiment 1 (not until 54 h). These results indicate that the removal of F. columnare from the water by A. anatina began after an initial delay. The reduction of bacterial concentration both in the mussel treatment and in the control group after 48 h in the present study could have been caused by, for example, an exhaustion of the nutrients required for steady bacterial growth, the bacteria’s attachment to the aquaria and shell surfaces, sedimentation or changes in water quality. However, the difference between the mussel treatment and control remained through the end of the experiment.
Flavobacterium columnare has been isolated from mussel tissue in nature (Starliper et al., 2008), but the ability of F. columnare to infect A. anatina has not been demonstrated. The present study isolated F. columnare from mussel gonad tissue in Experiment 1, but not in Experiment 2. In Experiment 1, we performed the intestine sampling before the gonad sampling after the gonad/intestine complex was cut open, and therefore cannot rule out the possibility that presence of F. columnare in gonad was due to contamination from intestine. Therefore, the infection of the mussel gonads by F. columnare in Experiment 1 is, to some degree, uncertain. Flavobacterium columnare’s ability to infiltrate mussel tissue must be studied more closely in the future. Flavobacterium columnare’s ability to infect the mussels is an important question that can determine whether mussels can act as transient reservoirs for accumulated bacteria, potentially increasing the risk of the pathogen’s spread (Ben-Horin et al., 2015; Bighiu et al., 2019).
There was no difference in the concentrations of algal cells between the experimental (with mussel) and control groups (without mussel), suggesting that the mussels did not significantly reduce the algal cell concentration. This was surprising and against the Hypothesis 2, especially when the biodeposition formation scores were high in the algal cell treatment. Algal cells tend to aggregate and accumulate at the bottom of the water (Berg et al., 1996). In addition, the algae used in the present study were preserved algae of marine origin, and it is possible that they started to break up in the hypotonic freshwater environment, which could also have contributed to the observed decrease in algae concentration. The results from the bacterial treatment indicated that the mussels’ filtration did not begin until after 24 h (Fig. 1A). This may have been too long a period of time for the preserved microalgae to maintain a good condition.
Mussels are highly sensitive to changes in their environment; therefore, mussel behavior can be used to monitor environmental conditions (Hartmann et al., 2015). In the present experiments, A. anatina behavior (shell openness, foot position, and movement) did not differ when the mussels were kept with bacteria, algal cells, or clean water, suggesting that the observed reduction of bacteria in the mussel treatment could not be explained by differences in the mussels’ behavior. Freshwater mussels mainly hold their shells open for feeding and respiration and keep them closed for long periods under stressful conditions (Englund & Heino, 1994). Thus, shell openness, which also measured filtration activity in terms of siphon exposure, suggests that no marked differences in the activity of mussels took place between the treatment groups. This may imply that the other ecosystem services provided by mussels including nutrient excretion and bioturbation would not be hindered during the bacterial removal.
A very high proportion of mussels in both experiments were infected with trematodes, mostly the bucephalid Rhipidocotyle fennica (Taskinen et al., 1991; Gibson et al., 1992). Rhipidocotyle fennica infection has many adverse effects on A. anatina (Jokela et al., 1993, 2005; Taskinen et al., 1994; Taskinen & Valtonen, 1995; Taskinen, 1998). There is no information available about the influence of trematode parasitism on mussels’ filtration rates; however, even if such an influence did exist, it would not skew the present results because all the treatment groups were equally trematode infected.
Bivalve mollusks consume a variety of particles like phytoplankton, organic detritus, and possibly dissolved organic matter (Ben-Horin et al., 2015). Phytoplankton is a primary food source for the majority of bivalves, but mussels can also utilize bacteria as a source of energy, surviving for long periods of time without any other food sources (Govorin, 2000; Gosling, 2008; Strayer, 2008; Ben-Horin et al., 2015; Burge et al., 2016). A high C:N ratio in the phytoplankton can meet mussels’ carbon requirements (Muir et al., 1986), while bacteria with a low C:N ratio as a food source provide them with the nitrogen they require (Seiderer et al., 1984). Therefore, it is not surprising that bivalve mollusks have been found to filter bacteria (e.g., Jørgensen et al., 1984; Muir et al., 1986; Kryger & Riisgård, 1988; Cotner et al., 1995; Silverman et al., 1995, 1997; Vanderploeg et al., 1995; Ben-Horin et al., 2015; Ismail et al., 2015; Burge et al., 2016; Mezzanotte et al., 2016). Indeed, finding F. columnare in the A. anatina intestines indicates that the mussels ingested the bacteria, suggesting that F. columnare could even be used as a food source by A. anatina, although this must be verified in further studies.
Filter-feeding bivalve mollusks often occur in dense beds; they provide many important ecological services (habitat modification, nutrient recycling and storage) and restorative functions through removing and concentrating suspended particles from the water column and improving water quality in aquatic ecosystems (Ben-Horin et al., 2015; Burge et al., 2016; Vaughn, 2018). Freshwater mussels filter large volumes of water and have shown their potential in water quality management as a tool for nutrient and particle removal (e.g., Strayer, 2014; Lummer et al., 2016; Mezzanotte et al., 2016; Hoellein et al., 2017). Therefore, they could be also used to decrease the environmental impact caused by organic wastes, to improve the hygienic condition of water, and to reduce pathogens and water-borne diseases (Jørgensen et al., 1984; Kryger & Riisgård, 1988; Cotner et al., 1995; Vanderploeg et al., 1995; Nichols & Garling, 2002; Christian et al., 2004; Mezzanotte et al., 2016), including E. coli (Silverman et al., 1995; Ismail et al., 2015).
The present study demonstrated the potential of A. anatina to remove the fish pathogen F. columnare from the water column in experimental conditions. Considering that F. columnare causes serious problems in fish farming (Wagner et al., 2002; Pulkkinen et al., 2010), it would be useful to investigate the efficacy of A. anatina in removing F. columnare at fish farms and their potential for reducing disease outbreaks in aquaculture. Anodonta anatina has been shown to markedly decrease the density of the cercarial larvae of the eye fluke Diplostomum pseudospathaceum (Trematoda), thereby decreasing the intensity of this harmful eye parasite in cultured rainbow trout (Gopko et al., 2017). Anodonta anatina’s potential to mitigate disease problems in aquaculture can be justified, as it reduces the density of two key fish pathogens—Flavobacterium and Diplostomum—whose combined co-infection effect on fish mortality is even higher than in single infections (Louhi et al., 2015).
The present finding of A. anatina’s bacteria-removing capabilities may indicate a capacity to regulate bacterial communities. Based on the current results, it is not possible to determine whether A. anatina can significantly influence natural F. columnare populations or reduce pathogen transmission and disease outbreaks in field conditions. Overall, the ecosystem effect of freshwater mussels through bacteria removal is largely unknown.
The present experimental results showed a significant reduction of F. columnare concentration in the water column by A. anatina, which is a significant finding with regard to the potential of mussels in mitigation of disease outbreaks in aquaculture. Although our experimental setup does not allow indicating the exact mechanism for the bacterial reduction, the presence of the bacteria in the mussels’ digestive systems suggests ingestion of bacteria by mussels. However, as there are challenges in extrapolating effects from laboratory or mesocosm experiments to field conditions, further studies are required to evaluate 1) the potential role of A. anatina, or freshwater mussels in general, in controlling natural bacterial populations; 2) the role of freshwater mussels in influencing the emergence and outbreaks of F. columnare disease in natural waters and in aquaculture; and 3) the possible role of bacteria as a food source of mussels. The present results indicate the potentially important, but poorly understood, effect of filter-feeding freshwater mussels on aquatic bacterial communities. Thus, freshwater mussels can provide potent ecosystem services including filtering and removing pathogens from water, which could be utilized in aquacultures and water quality management.
Data Availability
All the data and materials will be provided, if requested.
Code availability
Not applicable.
References
Ben-Horin, T., G. Bidegain, L. Huey, D. A. Narvaez & D. Bushek, 2015. Parasite transmission through suspension feeding. Journal of Invertebrate Pathology 131: 155–176.
Berg, D. J., S. W. Fisher & P. F. Landrum, 1996. Clearance and processing of algal particles by zebra mussels (Dreissena polymorpha). Journal of Great Lakes Research 22: 779–788.
Bighiu, M. A., A. Norman Haldén, W. Goedkoop & J. Ottoson, 2019. Assessing microbial contamination and antibiotic resistant bacteria using zebra mussels (Dreissena polymorpha). Science of the Total Environment 650: 2141–2149.
Bogan, A. E., 2008. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 595: 139–147.
Bontes, B. M., A. M. Verschoor, L. M. Dionisio Pires, E. van Donk & B. W. Ibelings, 2007. Functional response of Anodonta anatina feeding on a green alga and four strains of cyanobacteria, differing in shape, size and toxicity. Hydrobiologia 584: 191–204.
Burge, C. A., C. J. Closek, C. S. Friedman, M. L. Groner, C. M. Jenkins, A. Shore-Maggio & J. E. Welsh, 2016. The use of filter-feeders to manage disease in a changing world. Integrative and Comparative Biology 56: 573–587.
Christian, A., B. Smith, D. Berg, J. Smoot & R. Findlay, 2004. Trophic position and potential food sources of 2 species of unionid bivalves (Mollusca: Unionidae) in 2 small Ohio streams. Journal of the North American Benthological Society 23: 101–113.
Cilia, J. & F. Fratini, 2018. Antimicrobial properties of terrestrial snail and slug mucus. Journal of Complementary and Integrative Medicine 15: 3.
Clements, J. C. & L. A. Comeau, 2019. Nitrogen removal potential of shellfish aquaculture harvests in eastern Canada: A comparison of culture methods. Aquaculture Reports 13: 100–183.
Cotner, J. B., W. S. Gardner, J. R. Johnson, R. H. Sada, J. F. Cavaletto & R. T. Heath, 1995. Effects of Zebra Mussels (Dreissena polymorpha) on bacterioplankton: evidence for both size-selective consumption and growth stimulation. Journal of Great Lakes Research 21: 517–528.
Declercq, A. M., F. Haesebrouck, W. Van den Broeck, P. Bossier & A. Decostere, 2013. Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Veterinary Research 44: 27.
Decostere, A., F. Haesebrouck & L. A. Devriese, 1997. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. Journal of Clinical Microbiology 35: 322–324.
Englund, V. & M. Heino, 1994. Valve movement of Anodonta anatina and Unio tumidus (Bivalvia, Unionidae) in a eutrophic lake. Annales Zoologici Fennici Finnish Zoological and Botanical Publishing Board 31: 257–262.
Faust, C., D. Stallknecht, D. Swayne & J. Brown, 2009. Filter-feeding bivalves can remove avian influenza viruses from water and reduce infectivity. Proceedings of the Royal Society B: Biological Sciences Royal Society 276: 3727–3735.
Ferreira-Rodríguez, N., Y. B. Akiyama, O. V. Aksenova, R. Araujo, M. Christopher Barnhart, Y. V. Bespalaya, A. E. Bogan, I. N. Bolotov, P. B. Budha, C. Clavijo, S. J. Clearwater, G. Darrigran, V. T. Do, K. Douda, E. Froufe, C. Gumpinger, L. Henrikson, C. L. Humphrey, N. A. Johnson, O. Klishko, M. W. Klunzinger, S. Kovitvadhi, U. Kovitvadhi, J. Lajtner, M. Lopes-Lima, E. A. Moorkens, S. Nagayama, K.-O. Nagel, M. Nakano, J. N. Negishi, P. Ondina, P. Oulasvirta, V. Prié, N. Riccardi, M. Rudzīte, F. Sheldon, R. Sousa, D. L. Strayer, M. Takeuchi, J. Taskinen, A. Teixeira, J. S. Tiemann, M. Urbańska, S. Varandas, M. V. Vinarski, B. J. Wicklow, T. Zając & C. C. Vaughn, 2019. Research priorities for freshwater mussel conservation assessment. Biological Conservation 231: 77–87.
Geist, J., 2010. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): a synthesis of conservation genetics and ecology. Hydrobiologia 644: 69–88.
Gibson, D. I., J. Taskinen & E. T. Valtonen, 1992. Studies on bucephalid digeneans parasitising molluscs and fishes in Finland. II. The description of Rhipidocotyle fennica n. sp. and its discrimination by principal components analysis. Systematic Parasitology 23: 67–79.
Gopko, M., E. Mironova, A. Pasternak, V. Mikheev & J. Taskinen, 2017. Freshwater mussels (Anodonta anatina) reduce transmission of a common fish trematode (eye fluke, Diplostomum pseudospathaceum). Parasitology 144: 1971–1979.
Gosling, E., 2008. Bivalve molluscs: biology, ecology and culture, John Wiley & Sons, New York:
Govorin, I. A., 2000. Role of bivalves in the depuration of seawaters contaminated by bacteria. Russian Journal of Marine Biology 26: 81–88.
Haag, W. & J. Williams, 2014. Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735: 45–60.
Hartmann, J., S. Beggel, K. Auerswald, B. Stoeckle & J. Geist, 2015. Establishing mussel behavior as a biomarker in ecotoxicology. Aquatic Toxicology 170: 279–288.
Hoellein, T. J., C. B. Zarnoch, D. A. Bruesewitz & J. DeMartini, 2017. Contributions of freshwater mussels (Unionidae) to nutrient cycling in an urban river: filtration, recycling, storage, and removal. Biogeochemistry 135: 307–324.
Howard, J. K. & K. M. Cuffey, 2006. The functional role of native freshwater mussels in the fluvial benthic environment. Freshwater Biology 51: 460–474.
Ismail, N. S., H. Dodd, L. M. Sassoubre, A. J. Horne, A. B. Boehm & R. G. Luthy, 2015. Improvement of urban lake water quality by removal of Escherichia coli through the action of the bivalve Anodonta californiensis. Environmental Science & Technology 49: 1664–1672.
Jokela, J., J. Taskinen, P. Mutikainen & K. Kopp, 2005. Virulence of parasites in hosts under environmental stress: Experiments with anoxia and starvation. Oikos 108: 156–164.
Jokela, J., L. Uotila & J. Taskinen, 1993. Effect of the castrating trematode parasite Rhipidocotyle fennica on energy allocation of fresh-water clam Anodonta piscinalis. Functional Ecology 7: 332–338.
Jørgensen, C., T. Kørboe, F. Møhlenberg & H. Riisgård, 1984. Ciliary and mucus-net filter feeding, with special reference to fluid mechanical Characteristics. Marine Ecology Progress Series 15: 283–292.
Kotta, J., M. Futter, A. Kaasik, K. Liversage, M. Rätsep, F. R. Barboza, L. Bergström, P. Bergström, I. Bobsien, E. Díaz, K. Herkül, P. R. Jonsson, S. Korpinen, P. Kraufvelin, P. Krost, O. Lindahl, M. Lindegarth, M. M. Lyngsgaard, M. Mühl, A. N. Sandman, H. Orav-Kotta, M. Orlova, H. Skov, J. Rissanen, A. Šiaulys, A. Vidakovic & E. Virtanen, 2020. Cleaning up seas using blue growth initiatives: Mussel farming for eutrophication control in the Baltic Sea. Science of the Total Environment 709: 136–144.
Kreeger, D. A., C. M. Gatenby & P. W. Bergstrom, 2018. Restoration potential of several native species of bivalve molluscs for water quality improvement in mid-Atlantic watersheds. Journal of Shellfish Research 37: 1121–1157.
Kryger, J. & H. U. Riisgård, 1988. Filtration rate capacities in six species of european freshwater bivalves. Oecologia 77: 34–38.
Kunttu, H. M. T., L.-R. Sundberg, K. Pulkkinen & E. T. Valtonen, 2012. Environment may be the source of Flavobacterium columnare outbreaks at fish farms. Environmental Microbiology Reports 4: 398–402.
Lopes-Lima, M., R. Sousa, A. Teixeira, S. Varandas, N. Riccardi, D. Aldridge & E. Froufe, 2016. Newly developed microsatellite markers for the pan-European duck mussel, Anodonta anatina: revisiting the main mitochondrial lineages. Aquatic Conservation Marine and Freshwater Ecosystems 26: 307–318.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K.-O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2017. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews 92: 572–607.
Louhi, K.-R., L.-R. Sundberg, J. Jokela & A. Karvonen, 2015. Interactions among bacterial strains and fluke genotypes shape virulence of co-infection. Proceedings of the Royal Society B: Biological Sciences 282: 20152097.
Lummer, E.-M., K. Auerswald & J. Geist, 2016. Fine sediment as environmental stressor affecting freshwater mussel behavior and ecosystem services. Science of the Total Environment 571: 1340–1348.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, S. A. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, R. Hershler, K. E. Perez, B. Roth, M. Seddon, E. E. Strong & F. G. Thompson, 2004. The global decline of nonmarine mollusks. BioScience 54: 321–330.
McDonald, J. H., 2014. Handbook of Biological Statistics, 3rd ed. Sparky House Publishing, Baltimore, Maryland:
Mezzanotte, V., F. Marazzi, M. Bissa, S. Pacchioni, A. Binelli, M. Parolini, S. Magni, F. M. Ruggeri, C. De Giuli Morghen, C. Zanotto & A. Radaelli, 2016. Removal of enteric viruses and Escherichia coli from municipal treated effluent by zebra mussels. Science of the Total Environment 539: 395–400.
Muir, D. G., L. Seiderer, C. L. Davis, S. Painting & F. Robb, 1986. Filtration, lysis and absorption of bacteria by mussels Choromytilus meridionalis collected under upwelling and downwelling conditions. South African Journal of Marine Science 4: 169–179.
Nichols, S. J. & D. Garling, 2002. Evaluation of substitute diets for live algae in the captive maintenance of adult and subadult unionidae. Journal of Shellfish Research 21: 875–881.
Nielsen, P., P. Cranford, M. Maar & J. Petersen, 2016. Magnitude, spatial scale and optimization of ecosystem services from a nutrient extraction mussel farm in the eutrophic Skive Fjord, Denmark. Aquaculture Environment Interactions 8: 311–329.
Pulkkinen, K., L.-R. Suomalainen, A. F. Read, D. Ebert, P. Rintamäki & E. T. Valtonen, 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proceedings of the Royal Society B: Biological Sciences Royal Society 277: 593–600.
Rubiolo, J. A., L. M. Botana, & P. Martinez, 2019. Insights into Mussel Microbiome. Microbial Communities in Aquaculture Ecosystems 95–120.
R Development Core Team, 2015. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna:
Seiderer, L., C. Davis, F. Robb & R. Newell, 1984. Utilisation of bacteria as nitrogen resource by kelp-bed mussel Choromytilus meridionalis. Marine Ecology Progress Series 15: 109–116.
Silverman, H., E. C. Achberger, J. W. Lynn & T. H. Dietz, 1995. Filtration and utilization of laboratory-cultured bacteria by Dreissena polymorpha, Corbicula fluminea, and Carunculina texasensis. The Biological Bulletin 189: 308–319.
Silverman, H., S. J. Nichols, J. S. Cherry, E. Achberger, J. W. Lynn & T. H. Dietz, 1997. Clearance of laboratory-cultured bacteria by freshwater bivalves: differences between lentic and lotic unionids. Canadian Journal of Zoology 75: 1857–1866.
Starliper, C. E., R. J. Neves, S. D. Hanlon & P. Whittington, 2008. A survey of the indigenous microbiota (bacteria) in three species of mussels from the Clinch and Holston Rivers, Virginia. Journal of Shellfish Research 27: 1311–1317.
Strayer, D. L., 2008. Freshwater mussel ecology: a multifactor approach to distribution and abundance, University of California Press, Berkeley:
Strayer, D. L., 2014. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 735: 277–292.
Taskinen, J., 1998. Influence of trematode parasitism on the growth of a bivalve host in the field. International Journal for Parasitology 28: 599–602.
Taskinen, J., T. Mäkelä & E. T. Valtonen, 1997. Exploitation of Anodonta piscinalis (Bivalvia) by trematodes: parasite tactics and host longevity. Annales Zoologici Fennici 34: 37–46.
Taskinen, J. & E. T. Valtonen, 1995. Age-, size-, and sex-specific infection of Anodonta piscinalis (Bivalvia: Unionidae) with Rhipidocotyle fennica (Digenea: Bucephalidae) and its influence on host reproduction. Canadian Journal of Zoology 73: 887–897.
Taskinen, J., E. T. Valtonen & D. I. Gibson, 1991. Studies on bucephalid digeneans parasitising molluscs and fishes in Finland I. Ecological data and experimental studies. Systematic Parasitology 19: 81–94.
Taskinen, J., E. T. Valtonen & T. Mäkelä, 1994. Quantity of sporocysts and seasonality of two Rhipidocotyle species (Digenea: Bucephalidae) in Anodonta piscinalis (Mollusca: bivalvia). International Journal for Parasitology 24: 877–886.
Taylor, D., C. Saurel, P. Nielsen & J. K. Petersen, 2019. Production characteristics and optimization of mitigation mussel culture. Frontiers in Marine Science Frontiers 6: 698.
Tuttle-Raycraft, S. & J. Ackerman, 2018. Does size matter? Particle size vs. quality in bivalve suspension feeding. Freshwater Biology 63: 1560–1568.
Vanderploeg, H., J. R. Liebig & T. F. Nalepa, 1995. From picoplankton to microplankton: temperature-driven filtration by the unionid bivalve Lampsilis radiata siliquoidea in Lake St. Clair. Canadian Journal of Fisheries and Aquatic Sciences 52: 63–74.
Vaughn, C. C., 2018. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C., S. Nichols & D. Spooner, 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society 27: 409–423.
Wagner, B. A., D. J. Wise, L. H. Khoo & J. S. Terhune, 2006. The Epidemiology of bacterial diseases in food-size channel catfish. Journal of Aquatic Animal Health 18: 263–272.
Acknowledgements
The authors would like to thank Juha Ahonen, Ahti Karusalmi, and Nina Honkanen for their technical assistance in the laboratory and Hanna Suonia and the staff of Konnevesi Research Station (JYU) for their maintenance of the mussels. The Maj and Tor Nessling Foundation (SA and MH via KP), the EU European Neighbourhood Instrument (ENI) Kolarctic Cross-Border Collaboration (CBC) Program (Project SALMUS/KO1017; JT), and the Biological and Environmental Science Doctoral School of the JYU (MH) provided financial support for the study.
Funding
Open Access funding provided by University of Jyväskylä (JYU). The Maj and Tor Nessling Foundation (SA and MH via KP), the EU European Neighbourhood Instrument (ENI) Kolarctic Cross-Border Collaboration (CBC) Program (Project SALMUS/KO1017; JT), and the Biological and Environmental Science Doctoral School of the JYU (MH) provided financial support for the study.
Author information
Authors and Affiliations
Contributions
MH, SA, KP, and JT all conceived of and designed the experiments, performed the experiments, and collected the data. MH, SA, and KP analyzed the data. MH and SA wrote the first draft of the manuscript. Thereafter, MH, SA, KP, and JT contributed to writing the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
All authors have given their consent to participate.
Consent for publication
All authors have given their consent to participate.
Additional information
Handling editor: Eric R. Larson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajisafarali, M., Aaltonen, S., Pulkkinen, K. et al. Does the freshwater mussel Anodonta anatina remove the fish pathogen Flavobacterium columnare from water?. Hydrobiologia 849, 1067–1081 (2022). https://doi.org/10.1007/s10750-021-04769-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04769-6