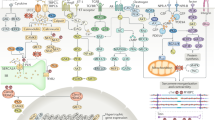
Abstract
Heart failure is one of the leading causes of death, with high mortality rate within 5 years after diagnosis. Treatment and prognosis options for heart failure primarily targeted on hemodynamic and neurohumoral components that drive progressive deterioration of the heart. However, given the multifactorial background that eventually leads to the “phenotype” named heart failure, better insight into the various components may lead to personalized treatment opportunities. Indeed, currently used criteria to diagnose and/or classify heart failure are possibly too focused on phenotypic improvement rather than the molecular driver of the disease and could therefore be further refined by integrating the leap of molecular and cellular knowledge. The ambiguity of the ejection fraction-based classification criteria became evident with development of advanced molecular techniques and the dawn of omics disciplines which introduced the idea that disease is caused by a myriad of cellular and molecular processes rather than a single event or pathway. The fact that different signaling pathways may underlie similar clinical manifestations calls for a more holistic study of heart failure. In this context, the systems biology approach can offer a better understanding of how different components of a system are altered during disease and how they interact with each other, potentially leading to improved diagnosis and classification of this condition. This review is aimed at addressing heart failure through a multilayer approach that covers individually some of the anatomical, morphological, functional, and tissue aspects, with focus on cellular and subcellular features as an alternative insight into new therapeutic opportunities.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.

Similar content being viewed by others
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB (2014) Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131:e1–e294. doi:10.1161/cir.0000000000000152
Roger VL (2013) Epidemiology of heart failure. Circ Res 113(6):646–659. doi:10.1161/circresaha.113.300268
Segovia Cubero J, Alonso-Pulpón Rivera L, Peraira Moral R, Silva Melchor L (2004) Heart failure: etiology and approach to diagnosis. Rev Esp Cardiol (English Edition) 57(3):250–259. doi:10.1016/s1885-5857(06)60143-6
Figueroa MS, Peters JI (2006) Congestive heart failure: diagnosis, pathophysiology, therapy, and implications for respiratory care. Respir Care 51(4):403–412
Dhalla NS, Rangi S, Babick AP, Zieroth S, Elimban V (2012) Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev 17(4-5):671–681. doi:10.1007/s10741-011-9278-7
Jahangir E, De Schutter A, Lavie CJ (2014) The relationship between obesity and coronary artery disease. Transl Res 164(4):336–344. doi:10.1016/j.trsl.2014.03.010
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 62(16):e147–e239. doi:10.1016/j.jacc.2013.05.019
De Keulenaer GW, Brutsaert DL (2011) Systolic and diastolic heart failure are overlapping phenotypes within the heart failure spectrum. Circulation 123(18):1996–2005. doi:10.1161/CIRCULATIONAHA.110.981431
Garattini L, Curto A, Freemantle N (2015) Personalized medicine and economic evaluation in oncology: all theory and no practice? Exp Rev Pharmacoecon Outcomes Res 15(5):733–738. doi:10.1586/14737167.2015.1078239
Liu LCY, Voors AA, Valente MAE, van der Meer P (2014) A novel approach to drug development in heart failure: towards personalized medicine. Can J Cardiol 30(3):288–295. doi:10.1016/j.cjca.2013.12.005
Mesquita ET, Jorge AJL, Souza Junior CVD, Cassino JPP (2014) Systems biology applied to heart failure with normal ejection fraction. Arq Bras Cardiol 102(5):510–517. doi:10.5935/abc.20140062
Kemp CD, Conte JV (2012) The pathophysiology of heart failure. Cardiovasc Pathol 21(5):365–371. doi:10.1016/j.carpath.2011.11.007
Jackson G (2000) ABC of heart failure: pathophysiology. BMJ 320(7228):167–170. doi:10.1136/bmj.320.7228.167
Vasan RS (2003) Cardiac function and obesity. Heart 89(10):1127–1129. doi:10.1136/heart.89.10.1127
Vargas-Uricoechea H, Sierra-Torres CH (2014) Thyroid hormones and the heart. Horm Mol Biol Clin Invest 18(1):15–26. doi:10.1515/hmbci-2013-0059
Fortuno MA, Ravassa S, Fortuno A, Zalba G, Diez J (2001) Cardiomyocyte apoptotic cell death in arterial hypertension: mechanisms and potential management. Hypertension 38(6):1406–1412. doi:10.1161/hy1201.099615
MacIver DH, Dayer MJ, Harrison AJ (2013) A general theory of acute and chronic heart failure. Int J Cardiol 165(1):25–34. doi:10.1016/j.ijcard.2012.03.093
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259. doi:10.1056/NEJMoa052256
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. doi:10.1016/j.jacc.2013.02.092
Borbély A, van der Velden J, Papp Z, Bronzwaer JGF, Edes I, Stienen GJM, Paulus WJ (2005) Cardiomyocyte stiffness in diastolic heart failure. Circulation 111(6):774–781. doi:10.1161/01.cir.0000155257.33485.6d
van Heerebeek L, Borbély A, Niessen HWM, Bronzwaer JGF, van der Velden J, Stienen GJM, Linke WA, Laarman GJ, Paulus WJ (2006) Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113(16):1966–1973. doi:10.1161/circulationaha.105.587519
Gladden JD, Linke WA, Redfield MM (2014) Heart failure with preserved ejection fraction. Pflügers Arch Eur J Physiol 466(6):1037–1053. doi:10.1007/s00424-014-1480-8
Borlaug BA, Paulus WJ (2011) Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 32(6):670–679. doi:10.1093/eurheartj/ehq426
Gaasch WH, Zile MR (2011) Left ventricular structural remodeling in health and disease. J Am Coll Cardiol 58(17):1733–1740. doi:10.1016/j.jacc.2011.07.022
Kehat I, Molkentin JD (2010) Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122(25):2727–2735. doi:10.1161/circulationaha.110.942268
Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A (2006) Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol 38(11):1834–1840. doi:10.1016/j.biocel.2006.04.010
Oka T, Akazawa H, Naito AT, Komuro I (2014) Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 114(3):565–571. doi:10.1161/CIRCRESAHA.114.300507
Nattel S, Shiroshita-Takeshita A, Cardin S, Pelletier P (2005) Mechanisms of atrial remodeling and clinical relevance. Curr Opin Cardiol 20(1):21–25
Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM (2003) Electrical remodeling of the atria in congestive heart failure electrophysiological and electroanatomic mapping in humans. Circulation 108(12):1461–1468. doi:10.1161/01.CIR.0000090688.49283.67
Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol 102(4):279–297. doi:10.1007/s00395-007-0658-z
Provost J, Lee WN, Fujikura K, Konofagou EE (2011) Imaging the electromechanical activity of the heart in vivo. Proc Natl Acad Sci U S A 108(21):8565–8570. doi:10.1073/pnas.1011688108
Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK (2008) Twist mechanics of the left ventricle. J Am Coll Cardiol Img 1(3):366–376. doi:10.1016/j.jcmg.2008.02.006
Nakatani S (2011) Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound 19(1):1–6. doi:10.4250/jcu.2011.19.1.1
Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, Rakowski H (2008) Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiogr: Off Pub Am Soc Echocardiogr 21(6):675–683. doi:10.1016/j.echo.2007.10.021
Bertini M, Nucifora G, Marsan NA, Delgado V, van Bommel RJ, Boriani G, Biffi M, Holman ER, Van der Wall EE, Schalij MJ, Bax JJ (2009) Left ventricular rotational mechanics in acute myocardial infarction and in chronic (ischemic and nonischemic) heart failure patients. Am J Cardiol 103(11):1506–1512. doi:10.1016/j.amjcard.2009.02.010
Blessberger H, Binder T (2010) Non-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart 96(9):716–722. doi:10.1136/hrt.2007.141002
D’Hooge J, Barbosa D, Gao H, Claus P, Prater D, Hamilton J, Lysyansky P, Abe Y, Ito Y, Houle H, Pedri S, Baumann R, Thomas J, Badano LP (2016) Two-dimensional speckle tracking echocardiography: standardization efforts based on synthetic ultrasound data. Eur Heart J Cardiovasc Imaging 17(6):693–701. doi:10.1093/ehjci/jev197
Zeiher AM, Wollschlaeger H, Bonzel T, Kasper W, Just H (1987) Hierarchy of levels of ischemia-induced impairment in regional left ventricular systolic function in man. Circulation 76(4):768–776. doi:10.1161/01.cir.76.4.768
Palmieri V, Okin PM, Bella JN, Gerdts E, Wachtell K, Gardin J, Papademetriou V, Nieminen MS, Dahlof B, Devereux RB, Losartan Intervention For End-point reduction in h (2003) Echocardiographic wall motion abnormalities in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Hypertension 41(1):75–82. doi:10.1161/01.HYP.0000045081.54784.36
Abate E, Hoogslag GE, Leong DP, Bertini M, Antoni ML, Nucifora G, Joyce E, Holman ER, Siebelink HM, Schalij MJ, Bax JJ, Delgado V, Ajmone Marsan N (2014) Association between multilayer left ventricular rotational mechanics and the development of left ventricular remodeling after acute myocardial infarction. J Am Soc Echocardiogr: Off Pub Am Soc Echocardiogr 27(3):239–248. doi:10.1016/j.echo.2013.12.009
Nguyen TP, Qu Z, Weiss JN (2014) Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol 70:83–91. doi:10.1016/j.yjmcc.2013.10.018
de Jong S, van Veen TA, van Rijen HV, de Bakker JM (2011) Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 57(6):630–638. doi:10.1097/FJC.0b013e318207a35f
Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, Rothery S (2004) Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta Biomembr 1662(1-2):138–148. doi:10.1016/j.bbamem.2003.10.019
Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM (2001) Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 104(25):3069–3075. doi:10.1161/hc5001.100833
Jin H, Chemaly ER, Lee A, Kho C, Hadri L, Hajjar RJ, Akar FG (2009) Mechanoelectrical remodeling and arrhythmias during progression of hypertrophy. FASEB J 24(2):451–463. doi:10.1096/fj.09-136622
Ciarka A, van de Borne P, Pathak A (2008) Myocardial infarction, heart failure and sympathetic nervous system activity: new pharmacological approaches that affect neurohumoral activation. Expert Opin Investig Drugs 17(9):1315–1330. doi:10.1517/13543784.17.9.1315
Sousa-Pinto B, Ferreira-Pinto MJ, Santos M, Leite-Moreira AF (2014) Central nervous system circuits modified in heart failure: pathophysiology and therapeutic implications. Heart Fail Rev 19(6):759–779. doi:10.1007/s10741-014-9427-x
Nijst P, Mullens W (2014) The acute cardiorenal syndrome: burden and mechanisms of disease. Curr Heart Fail Rep 11(4):453–462. doi:10.1007/s11897-014-0218-4
Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP (2014) Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J 35(7):448–454. doi:10.1093/eurheartj/eht456
Iwanaga Y, Kihara Y, Takenaka H, Kita T (2006) Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II–angiotensin type 1 receptor system. J Mol Cell Cardiol 41(5):798–806. doi:10.1016/j.yjmcc.2006.07.004
Reed BN, Street SE, Jensen BC (2014) Time and technology will tell: the pathophysiologic basis of neurohormonal modulation in heart failure. Heart Fail Clin 10(4):543–557. doi:10.1016/j.hfc.2014.07.002
Jonsson S, Agic MB, Narfstrom F, Melville JM, Hultstrom M (2014) Renal neurohormonal regulation in heart failure decompensation. Am J Physiol Regul Integr Comp Physiol 307(5):R493–R497. doi:10.1152/ajpregu.00178.2014
Lehmann LH, Stanmore DA, Backs J (2014) The role of endothelin-1 in the sympathetic nervous system in the heart. Life Sci 118(2):165–172. doi:10.1016/j.lfs.2014.03.005
Fujiu K, Nagai R (2013) Contributions of cardiomyocyte-cardiac fibroblast-immune cell interactions in heart failure development. Basic Res Cardiol 108(4):357. doi:10.1007/s00395-013-0357-x
Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S (2014) Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res 114(5):872–888. doi:10.1161/CIRCRESAHA.114.302533
Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I (2007) p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446(7134):444–448. doi:10.1038/nature05602
Atance J, Yost MJ, Carver W (2004) Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol 200(3):377–386. doi:10.1002/jcp.20034
Kresh JY, Chopra A (2011) Intercellular and extracellular mechanotransduction in cardiac myocytes. Pflügers Arch Eur J Physiol 462(1):75–87. doi:10.1007/s00424-011-0954-1
Cecchi F, Sgalambro A, Baldi M, Sotgia B, Antoniucci D, Camici PG, Sciagra R, Olivotto I (2009) Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res 2(4):452–461. doi:10.1007/s12265-009-9142-5
Fujiu K, Wang J, Nagai R (2014) Cardioprotective function of cardiac macrophages. Cardiovasc Res 102(2):232–239. doi:10.1093/cvr/cvu059
Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z (2013) B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19(10):1273–1280. doi:10.1038/nm.3284
Li Y, Zhang C, Wu Y, Han Y, Cui W, Jia L, Cai L, Cheng J, Li H, Du J (2012) Interleukin-12p35 deletion promotes CD4 T-cell-dependent macrophage differentiation and enhances angiotensin II-induced cardiac fibrosis. Arterioscler Thromb Vasc Biol 32(7):1662–1674. doi:10.1161/atvbaha.112.249706
Anker SD, von Haehling S (2004) Inflammatory mediators in chronic heart failure: an overview. Heart 90(4):464–470
Matsumoto K, Ogawa M, J-i S, Hirata Y, Nagai R, Isobe M (2011) Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int Heart J 52(6):382–387. doi:10.1536/ihj.52.382
Chen L, Gong Q, Stice JP, Knowlton AA (2009) Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84(1):91–99. doi:10.1093/cvr/cvp181
Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S (2002) Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci 99(3):1259–1263. doi:10.1073/pnas.241655498
Kubli DA, Gustafsson AB (2012) Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111(9):1208–1221. doi:10.1161/circresaha.112.265819
Neubauer S (2007) The failing heart—an engine out of fuel. N Engl J Med 356(11):1140–1151. doi:10.1056/NEJMra063052
Kolwicz SC, Tian R (2011) Glucose metabolism and cardiac hypertrophy. Cardiovasc Res 90(2):194–201. doi:10.1093/cvr/cvr071
Li L, Louch William E, Niederer Steven A, Aronsen Jan M, Christensen G, Sejersted Ole M, Smith Nicolas P (2012) Sodium accumulation in SERCA knockout-induced heart failure. Biophys J 102(9):2039–2048. doi:10.1016/j.bpj.2012.03.045
Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S (2011) Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2:e244. doi:10.1038/cddis.2011.130
Schwarzer M, Osterholt M, Lunkenbein A, Schrepper A, Amorim P, Doenst T (2014) Mitochondrial reactive oxygen species production and respiratory complex activity in rats with pressure overload-induced heart failure. J Physiol 592(17):3767–3782. doi:10.1113/jphysiol.2014.274704
Andre L, Fauconnier J, Reboul C, Feillet-Coudray C, Meschin P, Farah C, Fouret G, Richard S, Lacampagne A, Cazorla O (2013) Subendocardial increase in reactive oxygen species production affects regional contractile function in ischemic heart failure. Antioxid Redox Signal 18(9):1009–1020. doi:10.1089/ars.2012.4534
Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie RH, Sim K, Gao XM, Drummond G, Sarwar M, Zhang YY, Dart AM, Du XJ (2011) Myocardial oxidative stress contributes to transgenic beta(2)-adrenoceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol 162(5):1012–1028. doi:10.1111/j.1476-5381.2010.01043.x
Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P (2000) Myocardial cell death in human diabetes. Circ Res 87(12):1123–1132
Qin F, Siwik DA, Pimentel DR, Morgan RJ, Biolo A, Tu VH, Kang YJ, Cohen RA, Colucci WS (2014) Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. AJP: Heart Circ Physiol 306(10):H1453–H1463. doi:10.1152/ajpheart.00084.2014
Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB (2005) Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail 11(6):473–480. doi:10.1016/j.cardfail.2005.01.007
Foussal C, Lairez O, Calise D, Pathak A, Guilbeau-Frugier C, Valet P, Parini A, Kunduzova O (2010) Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Lett 584(11):2363–2370. doi:10.1016/j.febslet.2010.04.025
Heusch G, Schulz R (2011) A radical view on the contractile machinery in human heart failure⁎⁎Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. J Am Coll Cardiol 57(3):310–312. doi:10.1016/j.jacc.2010.06.057
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113(6):709–724. doi:10.1161/CIRCRESAHA.113.300376
Ferrans V, Butany JW (1983) Ultrastructural pathology of the heart. In: Trump BFJR (ed) Diagnostic electron microscopy, vol 4. Wiley, New York, pp 319–473
Maron BJ, Ferrans VJ, Roberts WC (1975) Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am J Pathol 79(3):387–434
Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM (2010) Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285(37):28938–28945. doi:10.1074/jbc.M110.154948
Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, Carcache de Blanco E, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S (2008) Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103(12):1466–1472. doi:10.1161/circresaha.108.184457
Neef S, Maier LS (2013) Novel aspects of excitation-contraction coupling in heart failure. Basic Res Cardiol 108(4):360. doi:10.1007/s00395-013-0360-2
Eisner D, Caldwell J, Trafford A (2013) Sarcoplasmic reticulum Ca-ATPase and heart failure 20 years later. Circ Res 113(8):958–961. doi:10.1161/CIRCRESAHA.113.302187
Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110(6):705–712. doi:10.1161/01.CIR.0000137836.95625.D4
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104(2):551–558. doi:10.1152/japplphysiol.01200.2007
Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D (2014) Ca handling during excitation–contraction coupling in heart failure. Pflügers Arch Eur J Physiol 466(6):1129–1137. doi:10.1007/s00424-014-1469-3
Peinado MÁ, Hernández R, Peragón J, Ovelleiro D, Pedrosa JÁ, Blanco S (2014) Proteomic characterization of nitrated cell targets after hypobaric hypoxia and reoxygenation in rat brain. J Proteome 109:309–321. doi:10.1016/j.jprot.2014.07.015
Blanco S, Castro L, Hernandez R, Del Moral ML, Pedrosa JA, Martinez-Lara E, Siles E, Peinado MA (2007) Age modulates the nitric oxide system response in the ischemic cerebellum. Brain Res 1157:66–73. doi:10.1016/j.brainres.2007.01.141
Lokuta AJ (2005) Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation 111(8):988–995. doi:10.1161/01.cir.0000156461.81529.d7
Steinberg SF (2013) Oxidative stress and sarcomeric proteins. Circ Res 112(2):393–405. doi:10.1161/circresaha.111.300496
Mann DL (2005) Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail Rev 10(2):95–100. doi:10.1007/s10741-005-4636-y
Buyandelger B, Mansfield C, Knoll R (2014) Mechano-signaling in heart failure. Pflugers Arch - Eur J Physiol 466(6):1093–1099. doi:10.1007/s00424-014-1468-4
Knoll R, Linke WA, Zou P, Miocic S, Kostin S, Buyandelger B, Ku CH, Neef S, Bug M, Schafer K, Knoll G, Felkin LE, Wessels J, Toischer K, Hagn F, Kessler H, Didie M, Quentin T, Maier LS, Teucher N, Unsold B, Schmidt A, Birks EJ, Gunkel S, Lang P, Granzier H, Zimmermann WH, Field LJ, Faulkner G, Dobbelstein M, Barton PJ, Sattler M, Wilmanns M, Chien KR (2011) Telethonin deficiency is associated with maladaptation to biomechanical stress in the mammalian heart. Circ Res 109(7):758–769. doi:10.1161/CIRCRESAHA.111.245787
Yin Z, Ren J, Guo W (2015) Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochim Biophys Acta (BBA) - Mol Basis Dis 1852(1):47–52. doi:10.1016/j.bbadis.2014.11.003
Booz GW, Day JN, Baker KM (2002) Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol 34(11):1443–1453
Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S (1999) Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res 84(10):1127–1136
Carreno JE, Apablaza F, Ocaranza MP, Jalil JE (2006) Cardiac hypertrophy: molecular and cellular events. Rev Esp Cardiol 59(5):473–486
Karin M, Z-g L, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9(2):240–246. doi:10.1016/s0955-0674(97)80068-3
Nadruz W Jr, Kobarg CB, Kobarg J, Franchini KG (2004) c-Jun is regulated by combination of enhanced expression and phosphorylation in acute-overloaded rat heart. Am J Phys Heart Circ Phys 286(2):H760–H767. doi:10.1152/ajpheart.00430.2003
Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR (2001) Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res 89(12):1122–1129. doi:10.1161/hh2401.100742
Lee H-g, Chen Q, Wolfram JA, Richardson SL, Liner A, Siedlak SL, Zhu X, Ziats NP, Fujioka H, Felsher DW, Castellani RJ, Valencik ML, McDonald JA, Hoit BD, Lesnefsky EJ, Smith MA (2009) Cell cycle re-entry and mitochondrial defects in myc-mediated hypertrophic cardiomyopathy and heart failure. PLoS ONE 4(9):e7172. doi:10.1371/journal.pone.0007172
Taegtmeyer H, Sen S, Vela D (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci 1188:191–198. doi:10.1111/j.1749-6632.2009.05100.x
Pérez-Serra A, Toro R, Sarquella-Brugada G, de Gonzalo-Calvo D, Cesar S, Carro E, Llorente-Cortes V, Iglesias A, Brugada J, Brugada R, Campuzano O (2016) Genetic basis of dilated cardiomyopathy. Int J Cardiol 224:461–472. doi:10.1016/j.ijcard.2016.09.068
Garcia-Pavia P, Cobo-Marcos M, Guzzo-Merello G, Gomez-Bueno M, Bornstein B, Lara-Pezzi E, Segovia J, Alonso-Pulpon L (2013) Genetics in dilated cardiomyopathy. Biomark Med 7(4):517–533. doi:10.2217/bmm.13.77
McNally Elizabeth M, Barefield David Y, Puckelwartz Megan J (2015) The genetic landscape of cardiomyopathy and its role in heart failure. Cell Metab 21(2):174–182. doi:10.1016/j.cmet.2015.01.013
McNair WP, Sinagra G, Taylor MR, Di Lenarda A, Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L, Familial Cardiomyopathy Registry Research G (2011) SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 57(21):2160–2168. doi:10.1016/j.jacc.2010.09.084
Xiong Q, Cao Q, Zhou Q, Xie J, Shen Y, Wan R, Yu J, Yan S, Marian AJ, Hong K (2015) Arrhythmogenic cardiomyopathy in a patient with a rare loss-of-function KCNQ1 mutation. J Am Heart Assoc 4(1):e001526. doi:10.1161/JAHA.114.001526
Ramos-Kuri M, Rapti K, Mehel H, Zhang S, Dhandapany PS, Liang L, Garcia-Carranca A, Bobe R, Fischmeister R, Adnot S, Lebeche D, Hajjar RJ, Lipskaia L, Chemaly ER (2015) Dominant negative Ras attenuates pathological ventricular remodeling in pressure overload cardiac hypertrophy. Biochim Biophys Acta 1853(11 Pt A):2870–2884. doi:10.1016/j.bbamcr.2015.08.006
Wei BR, Martin PL, Hoover SB, Spehalski E, Kumar M, Hoenerhoff MJ, Rozenberg J, Vinson C, Simpson RM (2011) Capacity for resolution of Ras-MAPK-initiated early pathogenic myocardial hypertrophy modeled in mice. Comp Med 61(2):109–118
Koera K, Nakamura K, Nakao K, Miyoshi J, Toyoshima K, Hatta T, Otani H, Aiba A, Katsuki M (1997) K-ras is essential for the development of the mouse embryo. Oncogene 15(10):1151–1159. doi:10.1038/sj.onc.1201284
Mohamed BA, Barakat AZ, Zimmermann WH, Bittner RE, Muhlfeld C, Hunlich M, Engel W, Maier LS, Adham IM (2012) Targeted disruption of Hspa4 gene leads to cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 53(4):459–468. doi:10.1016/j.yjmcc.2012.07.014
Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE (2016) A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351(6273):617–621. doi:10.1126/science.aad3456
Shang LL, Pfahnl AE, Sanyal S, Jiao Z, Allen J, Banach K, Fahrenbach J, Weiss D, Taylor WR, Zafari AM, Dudley SC (2007) Human heart failure is associated with abnormal C-terminal splicing variants in the cardiac sodium channel. Circ Res 101(11):1146–1154. doi:10.1161/circresaha.107.152918
Ufret-Vincenty CA, Baro DJ, Lederer WJ, Rockman HA, Quinones LE, Santana LF (2001) Role of sodium channel deglycosylation in the genesis of cardiac arrhythmias in heart failure. J Biol Chem 276(30):28197–28203. doi:10.1074/jbc.M102548200
He Q, Feng Y, Wang Y (2015) Transient outward potassium channel: a heart failure mediator. Heart Fail Rev 20(3):349–362. doi:10.1007/s10741-015-9474-y
Kaab S, Nuss HB, Chiamvimonvat N, O’Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF (1996) Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res 78(2):262–273. doi:10.1161/01.res.78.2.262
Tomaselli GF, Marbán E (1999) Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42(2):270–283. doi:10.1016/s0008-6363(99)00017-6
Näbauer M, Kääb S (1998) Potassium channel down-regulation in heart failure. Cardiovasc Res 37(2):324–334. doi:10.1016/s0008-6363(97)00274-5
Kaab S, Näbauer M (2001) Diversity of ion channel expression in health and disease. Eur Heart J Suppl 3(suppl_K):K31–K40. doi:10.1016/S1520-765X(01)90004-5
Yang Y, Chen X, Margulies K, Jeevanandam V, Pollack P, Bailey BA, Houser SR (2000) L-Type Ca2+ channel α1c subunit isoform switching in failing human ventricular myocardium. J Mol Cell Cardiol 32(6):973–984. doi:10.1006/jmcc.2000.1138
Zhang ZS, Cheng HJ, Onishi K, Ohte N, Wannenburg T, Cheng CP (2005) Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther 315(3):1203–1211. doi:10.1124/jpet.105.089672
Kashihara T, Hirose M, Shimojo H, Nakada T, Gomi S, Hongo M, Yamada M (2014) β2-Adrenergic and M2-muscarinic receptors decrease basal t-tubular L-type Ca2+ channel activity and suppress ventricular contractility in heart failure. Eur J Pharmacol 724:122–131. doi:10.1016/j.ejphar.2013.12.037
Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD (2012) Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Investig 122(1):280–290. doi:10.1172/jci58227
Libby P (2001) Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 104(3):365–372. doi:10.1161/01.cir.104.3.365
Mitra A, Basak T, Ahmad S, Datta K, Datta R, Sengupta S, Sarkar S (2015) Comparative proteome profiling during cardiac hypertrophy and myocardial infarction reveals altered glucose oxidation by differential activation of pyruvate dehydrogenase E1 component subunit β. J Mol Biol 427(11):2104–2120. doi:10.1016/j.jmb.2014.10.026
Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, Denfield SW (2006) Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail 12(2):139–143. doi:10.1016/j.cardfail.2005.11.001
Rosenthal D, Chrisant MRK, Edens E, Mahony L, Canter C, Colan S, Dubin A, Lamour J, Ross R, Shaddy R, Addonizio L, Beerman L, Berger S, Bernstein D, Blume E, Boucek M, Checchia P, Dipchand A, Drummond-Webb J, Fricker J, Friedman R, Hallowell S, Jaquiss R, Mital S, Pahl E, Pearce B, Rhodes L, Rotondo K, Rusconi P, Scheel J, Pal Singh T, Towbin J (2004) International Society for Heart and Lung Transplantation: practice guidelines for management of heart failure in children. J Heart Lung Transplant 23(12):1313–1333. doi:10.1016/j.healun.2004.03.018
Abramov D, He KL, Wang J, Burkhoff D, Maurer MS (2011) The impact of extra cardiac comorbidities on pressure volume relations in heart failure and preserved ejection fraction. J Card Fail 17(7):547–555. doi:10.1016/j.cardfail.2011.03.010
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) (2012) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 33(14):1750–1757. doi:10.1093/eurheartj/ehr254
Acknowledgements
We would like to thank Ard Teisman, David J. Gallacher, and Bruce Damiano for reviewing the manuscript and Lic. Pablo Aiger Diaz for figure artwork design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Johnson & Johnson corporate Integrated Technology Strategy (ITS) grant to Vijay Urmaliya.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Urmaliya, V., Franchelli, G. A multidimensional sight on cardiac failure: uncovered from structural to molecular level. Heart Fail Rev 22, 357–370 (2017). https://doi.org/10.1007/s10741-017-9610-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9610-y