Abstract
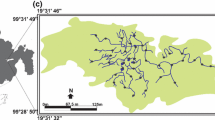
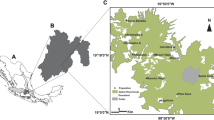
Human activities are affecting the distribution of species worldwide by causing fragmentation and isolation of populations. Isolation and fragmentation lead to populations with lower genetic variability and an increased chance of inbreeding and genetic drift, which results in a loss of biological fitness over time. Studies of the genetic structure of small and isolated populations are critically important for management and conservation decisions. Ambystoma rivulare is a micro-endemic Mexican mole salamander from central Mexico. It is found in the most ecologically disturbed region in Mexico, the Trans-Mexican Volcanic Belt. The goal of this study of the population genetics of the micro-endemic mole salamander was to provide information to be used as a basis for future research and conservation planning of this species and other species of the Ambystoma genus in Mexico. The structural analysis found two subpopulations, one for each river sampled, with no signs of admixture and very high levels of genetic differentiation. Medium to high levels of heterozygosity and few alleles and genotypes were observed. Evidence of an ancestral genetic bottleneck, low values of effective population size, small inbreeding coefficients, and low gene flow were also found.



Similar content being viewed by others
References
AmphibiaWeb (2016) Information on amphibian biology and conservation. AmphibiaWeb, Berkeley. http://amphibiaweb.org/. Accessed 20 Jan 2016
Arntzen JW, Smithson A, Oldham RS (1999) Marking and tissue sampling effects on body condition and survival in the newt Triturus cristatus. J Herpetol 33(4):567–576
Barriga-Vallejo C, Hernández-Gallegos O, Von-Herbing IH, López-Moreno AE, Ruiz-Gómez ML, Granados-González G, Garduño-Paz MV, Méndez-Sánchez JF, Banda-Leal J, Davis AK (2015) Assessing population health of the Toluca Axolotl Ambystoma rivulare (Taylor, 1940) from México using leukocyte profiles. Herpetol Conserv Biol 10(2):592–601
Beebee TJ, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125(3):271–285
Beerli P (2008) Migrate version 3.0: a maximum likelihood and Bayesian estimator of gene flow using the coalescent. http://popgen.sc.fsu.edu/Migrate/Migrate-n. Accessed 13 Nov 2014
Beerli P (2009) How to use migrate or why are Markov chain Monte Carlo programs difficult to use? In: Bertorelle G, Bruford MW, Hauffe HC, Rizzoli A, Vernesi C (eds) Population genetics for animal conservation: volume 17 of conservation biology. Cambridge University Press, Cambridge, pp 42–79
Beerli P (2012) Migrate documentation. http://popgen.sc.fsu.edu/migratedoc.pdf. Accessed 17 Aug 2015
Bradshaw CJA, Brook BW, Whiteman NK (2010) The conservation biologist’s toolbox—principles for the design and analysis of conservation studies. In: Sodhi NJ, Ehrlich PR (eds) Conservation biology for all. Oxford University Press, Great Britain, pp 330–333
Bryson RW, Linkem CW, Dorcas ME, Lathrop A, Jones JM, Alvarado-Díaz J, Grünwald CI, Murphy RW (2014) Multilocus species delimitation in the Crotalus triseriatus species group (Serpentes: Viperidae: Crotalinae) with the description of two new species. Zootaxa 3:475–496
Candeau DR, Franco MS (2007) Dinámica y condiciones de vida de la población del Parque Naciona Nevado de Toluca (PNNT) en la generación de presión a los sistemas circundantes. Investig Geogr 62:44–68
Casas-Andreu G, Cruz-Aviña R, Aguilar-Miguel X (2004) Un regalo poco conocido de México al mundo: el ajolote o axolotl (Ambystoma: Caudata: Amphibia) con algunas notas sobre la crítica situación de sus poblaciones. Cienc Ergo Sum 10(3):304–308
Castellano MJ, Valone TJ (2006) Effects of livestock removal and perennial grass recovery on the lizards of a desertified arid grassland. J Arid Environ 66:87–95
Champo-Jiménez O, Valderrama-Landeros L, España-Boqueros ML (2012) Forest cover loss in the Monarch Butterfly biosphere reserve, Michoacán, México (2006–2010). Rev Chapingo 18(2):143–157
CONAPO (2010) Delimitación de las zonas metropolitanas de México. http://www.conapo.gob.mx/en/CONAPO/Zonas_metro politanas_2010. June 20th, 2016
Cournet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent populations bottleneck from allele frequency data. Genetics 144:2001–2014
Crawford NG (2010) SMOGD: software for the measurement of genetic diversity. Mol Ecol Resour 10:556–557
Degne JF, Stout IJ, Roth JD, Parkinson CL (2007) Population genetics and conservation of the threatened southeastern beach mouse (Peromyscus polionotus niveiventris): subspecies and evolutionary units. Conserv Genet 8:1441–1452
Diario Oficial de la Federación Mexicana (DOF) (2013) DECRETO que reforma, deroga y adiciona diversas disposiciones del diverso publicado el 25 de enero de 1936, por el que se declaró Parque Nacional la montaña denominada “Nevado de Toluca” que fue modificado por el diverso publicado el 19 de febrero de 1937. México
Dlugosh KM, Parker M (2008) Founding events in species invasions: genetic variation adaptive evolution and the role of multiple introductions. Mol Ecol 17:431–449
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator 2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Earl DA, vonHoldt BM (2011) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Ellis EA, Porter-Bolland L (2008) Is community-based forest management more effective than protected areas?: a comparison of land use/land cover change in two neighboring study areas of the Central Yucatan Peninsula, Mexico. Forest Ecol Manag 256(11):1971–1983
Espindola S, Cuarón AD, Gaggiotti OE, Vázquez-Domínguez E (2014) High genetic structure of the Cozumel Harvest mice, a critically endangered island endemic: conservation implications. Conserv Genet 15:1393–1402
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
FAO Statistics database (2006). http://faostat.fao.org/. Accessed 10 June 2016
Flores-Villela O, García-Vázquez UO (2014) Biodiversidad de reptiles en México. Rev Mex Biodivers 85:467–475
Frankham R, Ballou J, Briscoe D (2005) Introduction to conservation genetics. Cambridge University Press, Cambridge
Frías-Alvarez P, Vance TV, Familiar-López M, Longocore JE, González-Bernal E, Santos-Barrera G, Zambrano L, Parra-Olea G (2008) Chytridiomycosis survey in wild and captive Mexican amphibians. Eco Health 5:18–26
Funk WC, Dunlap WW (1999) Colonization of high-elevation lakes by long-toed salamanders (Ambystoma macrodactylum) after the extinction of introduced trout populations. Can J Zool 77:1759–1767
Gamble LR, McGarigal K, Jenkins CL, Timm BC (2006) Limitations of regulated “buffer zones” for the conservation of marbled salamanders. Wetlands 26:298–306
Gamble LR, McGarigal K, Compton BW (2007) Fidelity and dispersal in the pond-breeding amphibian Ambystoma opacum: implications for spatio-temporal population dynamics and conservation. Biol Conserv 139:247–257
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Goprenko D, Williams RN, DeWoody JA (2007) Reproductive and mating success in the small-mouthed salamander (Ambystoma texanum) estimated via microsatellite parentage analysis. Evol Biol 34:130–139
Goudet J (2002) FSTAT version 2.9. 3.2: a program to estimate and test gene diversities and fixation indices. Institute of Ecology, Lausanne
Greenwald KR, Gibbs HL, Waite AT (2009) Efficacy of land-cover models in predicting isolation of marbled salamander populations in a fragmented landscape. Conserv Biol 25:1232–1241
Guillot G, Mortier F, Estoup A (2005) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5(3):712–715
Hedrick P (2005) Genetics of populations. Jones and Bartlett, Sudbury
Huey RB (1982) Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH (eds) Biology of the reptilian (vol 12 Physiology C. Physiological Ecology, pp 25–91). Academic Press, New York
IUCN Red List of Threatened Species (2016) http://www.iucnredlist.org. 15th March 2015
Jehle R, Arntzen JW (2002) Microsatellite markers in amphibian conservation genetics. Herpetol J 12:1–9
Johnson JR, Johnson BB, Shaffer B (2010) Genotype and temperature affect locomotor performance in a tiger salamander hybrid swarm. Funct Ecol 24:1073–1080
Jost L (2008) GST and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Tree 17:230–241
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Lemos-Espinal JA, Smith GR, Ruíz ÁH, Ayala RM (2016) Stream use and population characteristics of the endangered salamander, Ambystoma altamirani, from the Arroyo Los Axolotes, State of Mexico, Mexico. Southwest Nat 61(1):28–32
Marsh DM, Trenham PC (2001) Metapopulation dynamics and amphibian conservation. Conserv Biol 15(1):40–49
Mastretta-Yanes A, Quadri-Barba P, Escalante T, Arredondo-Amezcua L, Piñero D (2014) Propuesta de cambios a la zonificación y modificaciones al Programa de Manejo del APFF Nevado de Toluca tras reunión de discusión con CONANP en diciembre 2013. México
Matschiner M, Salzburger W (2009) TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25:1982–1983
Monroy-Vilchis O, Zarco-González MM, Domínguez-Vega H, Sunny A (2015) Ambystoma leorae (Taylor, 1943): new records, natural history notes and threat status. Herpetozoa 27:166–168
Myers EM, Zamudio K (2004) Multiple paternity in an aggregate breeding amphibian: the effect of reproductive skew on estimates of male reproductive success. Mol Ecol 13:1951–1963
Naughton GP, Henderson CB, Foresman KR, McGraw RL (2000) Long-toed salamanders in harvested and intact Douglas-fir forests of western Montana. Ecol Appl 10:1681–1689
Nei M (1972) Genetic distance between populations. Am Nat 106:283–292
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data. J Mol Evol 19(2):153–170
Noël S, Lapointe FJ (2010) Urban conservation genetics: study of a terrestrial salamander in the city. Biol Conserv 143:2823–2831
Palsbøll P, Berube M, Allendorf F (2007) Identification of management units using population genetic data. Trends Ecol Evol 22:11–16
Parra-Olea G, García-París M, Wake DB (1999) Status of some populations of Mexican salamanders (Amphibia: Plethodontidae). Rev Biol Trop 47:217–223
Parra-Olea G, Recuero E, Zamudio KR (2007) Primer note: polymorphic microsatellite markers for Mexican salamanders of the genus Ambystoma. Mol Ecol Notes 7:818–820
Parra-Olea G, Zamudio KR, Recuero E, Aguilar-Miguel X, Huacuz D, Zambrano L (2012) Conservation genetics of threatened Mexican axolotls (Ambystoma). Anim Conserv 15(1):61–72
Parra-Olea G, Flores-Villela O, Mendoza-Almeralla C (2014) Biodiversidad de anfibios en México. Rev Mex Biodivers 85:460–466
Pauly GB, Bennett SH, Palis JG, Shaffer HB (2012) Conservation and genetics of the frosted flatwoods salamander (Ambystoma cingulatum) on the Atlantic coastal plain. Conserv Genet 13(1):1–7
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Percino-Daniel R, Recuero E, Vázquez-Domínguez E, Zamudio KR, Parra-Olea G (2016) All grown-up and nowhere to go: paedomorphosis and local adaptation in Ambystoma salamanders in the Cuenca Oriental of México. Biol J Linn Soc 118(3):582–597
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Polich RL, Searcy CA, Shaffer HE (2013) Effects of tail clipping on survivorship and growth of larval salamanders. J Wildl Manag 77(7):1420–1425
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Purrenhage JL, Niewiarowski PH, Moore FBG (2009) Population structure of spotted salamanders (Ambystoma maculatum) in a fragmented landscape. Mol Ecol 18:235–247
Queller DC, Goodnigh KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
Raymond M, Rousset F (1995) GENEPOP v 12: population genetics software for exact test and ecumenicism. J Hered 86:248–249
Ribeiro R, Santos X, Sillero N, Carretero MA, Llorente GA (2009) Biodiversity and land uses at a regional scale: is agriculture the biggest threat for reptile assemblages? Acta Oecol 35:327–334
Rueda Zozaya P, Mendoza-Martínez G, Martínez-Gómez D, Monroy-Vilchis O, Antonio-Godoy J, Sunny A, Palomares F, Chávez C, Herrera-Haro J (2016) Genetic variability and structure of jaguar (Panthera onca) in Mexican zoos. Genetica 144:59–69
Sarukhán J, Koleff P, Carabias J, Soberon J, Dirzo R (2009) Capital Natural de México. Síntesis Conocimiento actual, evaluación y perspectivas de la sustentabilidad. México: Comisión Nacional para el Uso y Conocimiento de la Biodiversidad
Savage WK, Fremier AK, Shaffer HB (2010) Landscape genetics of alpine Sierra Nevada salamanders reveals extreme population subdivision in space and time. Mol Ecol 19:3301–3314
Searcy CA, Shaffer HB (2008) Calculating biologically accurate mitigation credits: insights from the California tiger salamander. Conserv Biol 22:997–1005
SEMARNAT (2010) Norma Oficial Mexicana NOM-059- SEMAR- NAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. México: Diario Oficial de la Federación, 10 diciembre 2010
Semlitsch RD (2008) Differentiating migration and dispersal processes for pond-breeding amphibians. J Wildl Manag 72:260–267
Shaffer B, Huacaz D, Flores-Villela O, Parra-Olea G, Wake D, Papenfuss T (2008) Ambystoma rivulare. http://www.iucnredlist.org/details/59067/0. Accessed 17 Sept 2014
Spear S, Storfer A (2010) Anthropogenic and natural disturbance lead to differing patterns of gene flow in the Rocky Mountain tailed frog, Ascaphus montanus. Biol Conserv 143:778–786
Spear SF, Peterson CR, Matocq MD, Storfer A (2006) Molecular evidence for historical and recent population size reductions of tiger salamanders (Ambystoma tigrinum) in Yellowstone National Park. Conserv Genet 7(4):605–611
Storfer A, Eastman JM, Spear SF (2009) Modern molecular methods for amphibian conservation. Bioscience 59:559–571
Summitt SD (2009) Determination of dispersal patterns of the small-mouthed salamander (Ambystoma texanum) in Eagle Creek Park (Indianapolis, IN). B.Sc Thesis. Butler University
Sunny A, Monroy-Vilchis O, Fajardo V, Aguilera-Reyes U (2014a) Genetic diversity and structure of an endemic and critically endangered stream river salamander (Caudata: Ambystoma leorae) in Mexico. Conserv Genet 15:49–59
Sunny A, Monroy-Vilchis O, Reyna-Valencia C, Zarco-González MM (2014b) Microhabitat types promote the genetic structure of a micro-endemic and critically endangered mole salamander (Ambystoma leorae) of Central Mexico. PLoS ONE 9:e103595. doi:10.1371/journalpone0103595
Sunny A, Monroy-Vilchis O, Zarco-González MM, Mendoza-Martínez GD, Martínez-Gómez D (2015) Genetic diversity and genetic structure of an endemic Mexican Dusky Rattlesnake (Crotalus triseriatus) in a highly modified agricultural landscape: implications for conservation. Genetica 143:705–716
Tallmon DA, Funk WC, Dunlap WW, Allendorf FW (2000) Genetic differentiation among long-toed salamander (Ambystoma macrodactylum) populations. Copeia 2000:27–35
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org. Accessed 15 Oct 2015
Tennessen JA, Zamudio KR (2003) Early-male reproductive advantage multiple paternity and sperm storage in an amphibian aggregate breeder. Mol Ecol 12:1567–1576
Trenham PC, Shaffer HB (2005) Amphibian upland habitat use and its consequences for population viability. Ecol Appl 15:1158–1168
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vázquez-Domínguez E, Surárez-Atilano M, Booth W, González-Baca C, Cuarón AD (2012) Genetic evidence of a recent successful colonization of introduced species on islands: Boa constrictor imperator on Cozumel Island. Biol Invasions 14:2101–2116
Vega R, Vázquez-Domínguez E, Mejía-Puente A, Cuarón AD (2007) Unexpected high levels of genetic variability and the population structure of an island endemic rodent (Oryzomys couesi cozumelae). Biol Conserv 137(2):210–222
Vidal O, López-García J, Rendón-Salinas E (2014) Trends in deforestation and forest degradation after a decade of monitoring in the Monarch Butterfly Biosphere Reserve in Mexico. Conserv Biol 28(1):177–186
Vucetich JA, Waite TA (2000) Is one migrant per generation sufficient for the genetic management of fluctuating populations? Anim Conserv 3(3):261–266
Waltert M, Mardiastuti A, Muhlenberg M (2004) Effects of land use on bird species richness in Sulawesi, Indonesia. Conserv Biol 18:1339–1346
Wang IJ (2009) Fine-scale population structure in a desert amphibian: landscape genetics of the black toad (Bufo exsul). Mol Ecol 18:3847–3856
Wang IJ, Summers K (2010) Genetic structure is correlated with phenotypic divergence rather than geographic isolation in the highly polymorphic strawberry poison-dart frog. Mol Ecol 19:447–458
Wang IJ, Savage WK, Shaffer HB (2009) Landscape genetics and least-cost path analysis reveal unexpected dispersal routes in the California tiger salamander (Ambystoma californiense). Mol Ecol 18:1365–1374
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
White D, Minotti PG, Barczak MJ, Sifneos JC, Freemark KE, Santelmann MV, Steinitz CF, Kiester AR, Preston EM (1997) Assessing risks to biodiversity from future landscape change. Conserv Biol 11:349–360
WWF (2004) La tala ilegal y su impacto en la reserva de la biosfera Mariposa Monarca. World Wildlife Fund, México
Zambrano L, Valiente E, Vander Zanden MJ (2010) Food web overlap among native axolotl (Ambystoma mexicanum) and two exotic fishes: carp (Cyprinus carpio) and tilapia (Oreochromis niloticus) in Xochimilco, Mexico City. Biol Invasions 12(9):3061–3069
Acknowledgements
The Universidad Autónoma del Estado de México funded the study (3047-2011E). R.L.H-B is grateful to CONACYT and COMECYT (359990 and 16BTID0028) for scholarships. To the students of CICBA for helps us in data collect. We also thank the editor and two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heredia-Bobadilla, RL., Monroy-Vilchis, O., Zarco-González, M.M. et al. Genetic structure and diversity in an isolated population of an endemic mole salamander (Ambystoma rivulare Taylor, 1940) of central Mexico. Genetica 144, 689–698 (2016). https://doi.org/10.1007/s10709-016-9935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-016-9935-9