Abstract
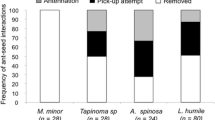
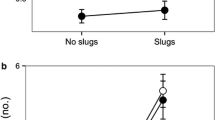
Myrmecochorous plants produce seeds with lipid-rich appendages (elaiosomes) which act as a reward for seed-dispersing ants. Seed dispersal is important for exotic species, which often need to establish new mutualistic interactions in order to colonize new non-native habitats. However, little is known about the importance of elaiosomes for seed removal in many of their non-native ranges. We studied ant–seed interactions of elaiosome-bearing and elaiosome-removed seeds of the Australian trees Acacia dealbata and Acacia longifolia in order to assess the relative importance of elaiosomes for seed removal between their native (Australia) and non-native (Portugal) ranges. In Portugal, we also studied the co-occurring native plant species with myrmecochorous seeds, Pterospartum tridentatum and Ulex europaeus, across three contiguous levels of acacia invasion: control (i.e. no acacia), low, and high acacia tree density. Acacia seeds were successfully removed by ants in their non-native region by a diversified assemblage of ant species, even in sites where native plants interacted with only one specialized ant species. In the invaded range, diminishing relative importance of elaiosomes was associated with changes in the ant community due to acacia invasion, and for A. dealbata, ant species richness decreased with increasing acacia tree density. Although seed removal was high for both acacia species, the importance of elaiosomes was proportionally lower for A. dealbata in the non-native region. Native plant species experienced significant reductions in seed removal in areas highly invaded by acacia, identifying another mechanism of displacement of native plants by acacias.


Similar content being viewed by others
References
Aguilera N, Becerra J, Villaseñor-Parada C et al (2015) Effects and identification of chemical compounds released from the invasive Acacia dealbata Link. Chem Ecol 31:479–493. https://doi.org/10.1080/02757540.2015.1050004
Aizen MA, Morales CL, Morales JM (2008) Invasive mutualists erode native pollination webs. PLoS Biol 6:e31. https://doi.org/10.1371/journal.pbio.0060031
Alba-Lynn C, Henk S (2010) Potential for ants and vertebrate predators to shape seed-dispersal dynamics of the invasive thistles Cirsium arvense and Carduus nutans in their introduced range (North America). Plant Ecol 210:291–301. https://doi.org/10.1007/s11258-010-9757-2
Anderson MJ, Crist TO, Chase JM et al (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686. https://doi.org/10.1890/03-0650
Auld TD (1986) Population dynamics of the shrub Acacia suaveolens (Sm.) Willd.: fire and the transition to seedlings. Aust J Ecol 11:373–385. https://doi.org/10.1111/j.1442-9993.1986.tb01407.x
Bartomeus I, Vilà M, Santamaría L (2008) Contrasting effects of invasive plants in plant-pollinator networks. Oecologia 155:761–770. https://doi.org/10.1007/s00442-007-0946-1
Bas JM, Oliveras J, Gómez C (2007) Final seed fate and seedling emergence in myrmecochorous Plants: effects of ants and plant species. Sociobiology 50:101–111. www.csuchico.edu/biol/Sociobiology/sociobiologyindex.html
Bas JM, Oliveras J, Gómez C (2009) Myrmecochory and short-term seed fate in Rhamnus alaternus: ant species and seed characteristics. Acta Oecol 35:380–384. https://doi.org/10.1016/j.actao.2009.02.003
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Blüthgen N, Menzel F, Blüthgen N (2006) Measuring specialization in species interaction networks. BMC Ecol 6:9. https://doi.org/10.1186/1472-6785-6-9
Bossard CC (1991) the role of habitat disturbance, seed predation and ant dispersal on establishment of the exotic shrub cytisus scoparius in California. Am Midl Nat 126:1–13. https://doi.org/10.2307/2426145
Burgos E, Ceva H, Perazzo RPJ et al (2007) Why nestedness in mutualistic networks? J Theor Biol 249:307–313. https://doi.org/10.1016/j.jtbi.2007.07.030
Buscardo E, Rodríguez-Echeverría S, Martín MP et al (2010) Impact of wildfire return interval on the ectomycorrhizal resistant propagules communities of a Mediterranean open forest. Fungal Biol 114:628–636. https://doi.org/10.1016/j.funbio.2010.05.004
Castro S, Ferrero V, Loureiro J et al (2009) Dispersal mechanisms of the narrow endemic Polygala vayredae: dispersal syndromes and spatio-temporal variations in ant dispersal assemblages. Plant Ecol 207:359–372. https://doi.org/10.1007/s11258-009-9679-z
Castroviejo S (2001) Claves de Flora Iberica: plantas vasculares de la Península Ibérica e Islas Baleares. Editorial CSIC—CSIC Press, Madrid
Castroviejo S (2012) Flora ibérica: plantas vasculares de la Península Ibérica e Islas Baleares. Real Jard¡n Botánico, C.S.I.C., Madrid
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9.1.0
Correia M, Castro S, Ferrero V et al (2014) Reproductive biology and success of invasive Australian acacias in Portugal. Bot J Linn Soc 174:574–588. https://doi.org/10.1111/boj.12155
Correia M, Montesinos D, French K, Rodríguez-Echeverría S (2016) Evidence for enemy release and increased seed production and size for two invasive Australian acacias. J Ecol 104:1391–1399. https://doi.org/10.1111/1365-2745.12612
Davidson DW, Morton SR (1984) Dispersal adaptations of some Acacia species in the Australian arid zone. Ecology 65:1038–1051
De Almeida JD, Freitas H (2006) Exotic naturalized flora of continental Portugal—a reassessment. Bot Complut 30:117–130
Dormann CF, Fruend J, Bluethgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24
Dunne JA, Williams RJ, Martinez ND (2002) Food-web structure and network theory: the role of connectance and size. Proc Natl Acad Sci USA 99:12917–12922. https://doi.org/10.1073/pnas.192407699
Ferrero V, Castro S, Costa J et al (2013) Effect of invader removal: pollinators stay but some native plants miss their new friend. Biol Invasions 15:2347–2358. https://doi.org/10.1007/s10530-013-0457-4
Fokuhl G, Heinze J, Poschlod P (2012) Myrmecochory by small ants—beneficial effects through elaiosome nutrition and seed dispersal. Acta Oecol 38:71–76. https://doi.org/10.1016/j.actao.2011.09.007
French K, Major RE (2001) Effect of an exotic Acacia (Fabaceae) on ant assemblages in South African fynbos. Austral Ecol 26:303–310. https://doi.org/10.1046/j.1442-9993.2001.01115.x
Gibson MR, Richardson DM, Marchante E et al (2011) Reproductive biology of Australian acacias: Important mediator of invasiveness? Divers Distrib 17:911–933. https://doi.org/10.1111/j.1472-4642.2011.00808.x
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492. https://doi.org/10.1111/j.0030-1299.2006.14258.x
Gómez C, Espadaler X (1998) Myrmecochorous dispersal distances: a world survey. J Biogeogr 25:573–580
Gómez C, Espadaler X (2013) An update of the world survey of myrmecochorous dispersal distances. Ecography (Cop) 36:1193–1201. https://doi.org/10.1111/j.1600-0587.2013.00289.x
Gorb EV, Gorb SN (1999) Dropping rates of elaiosome-bearing seeds during transport by ants (Formica polyctena Foerst.): implications for distance dispersal. Acta Oecol 20:509–518
Gove AD, Majer JD, Dunn RR (2007) A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 153:687–697. https://doi.org/10.1007/s00442-007-0756-5
Harris CJ, Manea A, Moles AT et al (2016) Differences in life-cycle stage components between native and introduced ranges of five woody Fabaceae species. Austral Ecol. https://doi.org/10.1111/aec.12456
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156. https://doi.org/10.1890/0012-9658(1999)080%5b1150:TMAORR%5d2.0.CO;2
Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15. https://doi.org/10.1111/j.1365-2745.2004.00953.x
Holmes PM (1990) Dispersal and predation in alien Acacia. Oecologia 83:288–290
Hughes L, Westoby M (1992) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312. https://doi.org/10.2307/1940677
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–586. https://doi.org/10.2307/1934145
IBM (2010) IBM SPSS Statistics for Windows
Jensen JM, Six DL (2006) Myrmecochory of the exotic plant, Centaurea maculosa: a potential mechanism enhancing invasiveness. Environ Entomol 35:326–331. https://doi.org/10.1603/0046-225X-35.2.326
Lach L, Parr CL, Abbott KL (2010) Ant ecology. Oxford University Press, Oxford
Lankau RA, Nuzzo V, Spyreas G, Davis AS (2009) Evolutionary limits ameliorate the negative impact of an invasive plant. Proc Natl Acad Sci USA 106:15362–15367. https://doi.org/10.1073/pnas.0905446106
Leal LC, Neto MCL, de Oliveira AFM et al (2014) Myrmecochores can target high-quality disperser ants: variation in elaiosome traits and ant preferences for myrmecochorous Euphorbiaceae in Brazilian Caatinga. Oecologia 174:493–500. https://doi.org/10.1007/s00442-013-2789-2
Lengyel S, Gove AD, Latimer AM et al (2009) Ants sow the seeds of global diversification in flowering plants. PLoS ONE 4:e5480. https://doi.org/10.1371/journal.pone.0005480
Lengyel S, Gove AD, Latimer AM et al (2010) Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspect Plant Ecol Evol Syst 12:43–55. https://doi.org/10.1016/j.ppees.2009.08.001
Lorenzo P, González L, Reigosa MJ (2010a) The genus Acacia as invader: the characteristic case of Acacia dealbata Link in Europe. Ann For Sci 67:101. https://doi.org/10.1051/forest/2009082
Lorenzo P, Palomera-Pérez A, Reigosa MJ, González L (2010b) Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol 212:403–412. https://doi.org/10.1007/s11258-010-9831-9
Lorenzo P, Pazos-Malvido E, Reigosa MJ, González L (2010c) Differential responses to allelopathic compounds released by the invasive Acacia dealbata Link (Mimosaceae) indicate stimulation of its own seed. Aust J Bot 58:546–553
Manzaneda AJ, Rey PJ (2008) Geographic variation in seed removal of a myrmecochorous herb: influence of variation in functional guild and species composition of the disperser assemblage through spatial and temporal scales. Ecography (Cop) 31:583–591. https://doi.org/10.1111/j.0906-7590.2008.05345.x
Manzaneda AJ, Rey PJ (2009) Assessing ecological specialization of an ant–seed dispersal mutualism through a wide geographic range. Ecology 90:3009–3022. https://doi.org/10.2307/25592842
Marchante H, Freitas H, Hoffmann JH (2010) Seed ecology of an invasive alien species, Acacia longifolia (Fabaceae), in Portuguese dune ecosystems. Am J Bot 97:1780–1790. https://doi.org/10.3732/ajb.1000091
Mark S, Olesen JM (1996) Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 107:95–101. https://doi.org/10.1007/BF00582239
Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proc Biol Sci 271:2605–2611. https://doi.org/10.1098/rspb.2004.2909
Montesinos D, Castro S, Rodríguez-Echeverría S (2012) Invasive acacias experience higher ant seed removal rates at the invasion edges. Web Ecol 12:33–37. https://doi.org/10.5194/we-12-33-2012
Montesinos D, Castro S, Rodríguez-Echeverría S (2016) Two invasive acacia species secure generalist pollinators in invaded communities. Acta Oecol 74:46–55. https://doi.org/10.1016/j.actao.2016.06.002
Pellow BJ, Henwood MJ, Carolin RC (2009) Flora of the Sydney region: a complete revision, 5th edn. Sydney University Press, Sydney
Pemberton RW, Irving DW (1990) Elaiosomes on weed seeds and the potential for myrmecochory in naturalized plants. Weed Sci 38:615–619
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803. https://doi.org/10.1038/439803a
Phillips BL, Brown GP, Greenlees M et al (2007) Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecol 32:169–176. https://doi.org/10.1111/j.1442-9993.2007.01664.x
Prior KM, Robinson JM, Dunphy SA, Frederickson ME (2015) Mutualism between co-introduced species facilitates invasion and alters plant community structure. Proc Biol Sci 282:20142846. https://doi.org/10.1098/rspb.2014.2846
R Development Core Team (2010) R: a language and environment for statistical computing. R Found Stat Comput Vienna Austria 0. ISBN 3-900051-07-0
Rejmánek M, Richardson DM (2013) Trees and shrubs as invasive alien species—2013 update of the global database. Divers Distrib 19:1093–1094. https://doi.org/10.1111/ddi.12075
Richardson DM, Allsopp N, D’Antonio CM et al (2000) Plant invasions—the role of mutualisms. Biol Rev Camb Philos Soc 75:65–93
Richardson DM, Carruthers J, Hui C et al (2011) Human-mediated introductions of Australian acacias—a global experiment in biogeography. Divers Distrib 17:771–787. https://doi.org/10.1111/j.1472-4642.2011.00824.x
Rodríguez-Echeverría S (2010) Rhizobial hitchhikers from down under: Invasional meltdown in a plant-bacteria mutualism? J Biogeogr 37:1611–1622. https://doi.org/10.1111/j.1365-2699.2010.02284.x
Rodríguez-Echeverría S, Crisóstomo JA, Nabais C, Freitas H (2009) Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions 11:651–661. https://doi.org/10.1007/s10530-008-9280-8
Rodríguez-Echeverría S, Fajardo S, Ruiz-Díez B, Fernández-Pascual M (2012) Differential effectiveness of novel and old legume–rhizobia mutualisms: implications for invasion by exotic legumes. Oecologia 170:253–261. https://doi.org/10.1007/s00442-012-2299-7
Rodríguez-Echeverría S, Afonso C, Correia M et al (2013) The effect of soil legacy on competition and invasion by Acacia dealbata Link. Plant Ecol 214:1139–1146. https://doi.org/10.1007/s11258-013-0238-2
Rodriguez-Girones MA, Santamaria L (2006) A new algorithm to calculate the nestedness temperature of presence-absence matrices. J Biogeogr 33:924–935. https://doi.org/10.1111/j.1365-2699.2006.01444.x
Sallabanks S (1993) Fruiting plant attractiveness to avian seed dispersers: native versus invasive Crataegus in Western Oregon. Madrono 40:108–116
Shattuck S (1999) Australian ants. Their biology and identification. CSIRO Publishing, Sydney
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: Invasional meltdown? Biol Invasions 1:21–32
Smith JMB (1989) An example of ant-assisted plant invasion. Aust J Ecol 14:247–250. https://doi.org/10.1111/j.1442-9993.1989.tb01433.x
Traveset A, Richardson D (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216. https://doi.org/10.1016/j.tree.2006.01.006
Traveset A, Richardson DM (2011) Mutualisms : Key drivers of invasions … key casualties of invasions. Fifty years invasion Ecol. Leg. Charles Elt. Blackwell Publishing Ltd, pp 143–160
Traveset A, Richardson DM (2014) Mutualistic interactions and biological invasions. Annu Rev Ecol Evol Syst 45:89–113. https://doi.org/10.1146/annurev-ecolsys-120213-091857
Werner C, Zumkier U, Beyschlag W, Máguas C (2009) High competitiveness of a resource demanding invasive acacia under low resource supply. Plant Ecol 206:83–96. https://doi.org/10.1007/s11258-009-9625-0
Whitney KD (2002) Dispersal for distance? Acacia ligulata seeds and meat ants Iridomyrmex viridiaeneus. Austral Ecol 27:589–595. https://doi.org/10.1046/j.1442-9993.2002.01216.x
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Williams PA, Karl BJ (2002) Birds and small mammals in kanuka (Kunzea ericoides) and gorse (Ulex europaeus) scrub and the resulting seed rain and seedling dynamics. N Z J Ecol 26:31–41
Willson MF, Traveset A (2000) The ecology of seed dispersal. In: Fenner M (ed) Seeds the ecology of regeneration plant communities, 2nd edn. CAB International, Wallingford, pp 85–110
Acknowledgements
Thanks to Andreia Jorge for help in the lab and to Steve Shattuck and Xavier Espadaler for confirmation of ant identification. Research was Funded by the Portuguese Fundação para a Ciência e a Tecnologia (FCT) via Project Mutualnet (PCT/BIA-BEC/103507/2008), co-founding by the EU via QREN, COMPETE and FEDER (UID/BIA/04004/2013), and scholarships and Grants to DM (SFRH/BPD/72595/2010; Starting grant IF/00066/2013), SC (Starting Grant IF/01267/2013), and SRE (Development Grant IF/00462/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendices
Appendix 1: Rarefaction curves
See Fig. 3.
Appendix 2: Interaction networks
See Fig. 4 and Tables 3, 4 and 5.
To visualize the relative strength of interactions on each site, we calculated ant–seed interaction networks for each of the two Portuguese sites with the statistical package “bipartite” on R 3.1.2 (Dormann et al. 2009). For each network, we calculated the following indexes: connectance, indicative of the realized proportion of possible links, obtained by the total sum of links divided by the number of cells in the interaction matrix (Dunne et al. 2002); nestedness (weighted NODF) indicating how the system is organized, with values closest to zero indicating high nestedness and values nearing 100 low nestedness (Rodriguez-Girones and Santamaria 2006); and network specialization index H2′, describing the level of specialization of the network, and ranging from zero (no specialization) to 1 (complete specialization) (Blüthgen et al. 2006); animal robustness, measuring the sensitivity of the system to the loss of plant species; and plant robustness, measuring the sensitivity of the system to the loss of animal species (Memmott et al. 2004; Burgos et al. 2007). For detailed descriptions of the different indexes used, see Dormann et al. (2009) and references therein. The study of interaction networks requires at least two species at each interaction level. Since we only had one plant species in Australia, we could only calculate interaction indexes for the Portuguese populations, for which two plant species were considered. However, we plotted interaction graphs for the Australian observations to quantitatively compare their interaction strength to those of Portugal.
Rights and permissions
About this article
Cite this article
Montesinos, D., Correia, M., Castro, S. et al. Diminishing importance of elaiosomes for acacia seed removal in non-native ranges. Evol Ecol 32, 601–621 (2018). https://doi.org/10.1007/s10682-018-9959-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-018-9959-y