Abstract
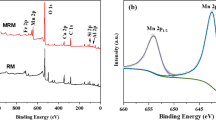
This study used red mud modified with chitosan (RM/CS) as a novel adsorbent to remove Ni(II) ions from an aqueous solution. The adsorbent was characterized by the techniques of the BET method, X-ray diffraction (XRD), and scanning electron microscopy (SEM) analysis. According to the findings, the surface area of RM/CS is nearly doubled compared to CS, from 68.6 to 105.7 m2.g−1. The Ni(II) batch adsorption of RM/CS was performed as a function of pH value, contact time, and volume of adsorbent. Three isotherm adsorption models (Langmuir, Freundlich, and Sips) and three kinetic models (the pseudo-first-order, the pseudo-second-order, and the intra-diffusion models) were fitted with the experimental data to calculate the maximum adsorption capacity and to estimate the uptake in nature. The Langmuir monolayer adsorption capacity for Nickel (II) is 31.66 mg.g−1 at a pH of 6.0, with an adsorption time of 180 min and a temperature of 323 K. The Ni(II) adsorption on RM/CS is the exothermic process and is controlled by the intra-diffusion model.






Similar content being viewed by others
Data availability
Not applicable.
References
Aigbe, U. O., et al. (2021). Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. Journal of Materials Research and Technology, 14, 2751–2774.
Al-Ghouti, M. A., & Al-Absi, R. S. (2020). Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Scientific Reports, 10, 15928.
Al-Ghouti, M. A., & Da’ana, D. A. (2020). Guidelines for the use and interpretation of adsorption isotherm models: A review. Journal of Hazardous Materials, 393, 122383.
Bartczak, P., et al. (2018). Removal of nickel (II) and lead (II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arabian Journal of Chemistry, 11, 1209–1222.
Bayramoglu, G., Altintas, B., & Arica, M. Y. (2009). Adsorption kinetics and thermodynamic parameters of cationic dyes from aqueous solutions by using a new strong cation-exchange resin. Chemical Engineering Journal, 152, 339–346.
Çelebi, H., Gök, G., & Gök, O. (2020). Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Scientific Reports, 10, 17570.
de Jesus, C. P. C., et al. (2015). Removal of reactive dye from aqueous solution using thermally treated red mud. Desalination and Water Treatment, 55, 1040–1047.
Deng, X., Qi, L., & Zhang, Y. (2018). Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash. Water, Air, & Soil Pollution, 229, 18.
Dinh, V.-P., et al. (2020a). Chitosan-MnO2 nanocomposite for effective removal of Cr (VI) from aqueous solution. Chemosphere, 257, 127147.
Dinh, V.-P., et al. (2020b). Primary biosorption mechanism of lead (II) and cadmium (II) cations from aqueous solution by pomelo (Citrus maxima) fruit peels. Environmental Science and Pollution Research, 28, 63504–63515.
Ewecharoen, A., Thiravetyan, P., & Nakbanpote, W. (2008). Comparison of nickel adsorption from electroplating rinse water by coir pith and modified coir pith. Chemical Engineering Journal, 137, 181–188.
Feng, N., et al. (2011). Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. Journal of Hazardous Materials, 185, 49–54.
Hamza, M. F., et al. (2019). Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chemical Engineering Journal, 362, 310–324.
Hannachi, Y., Shapovalov, N. A., & Hannachi, A. (2009). Adsorption of nickel from aqueous solution by the use of low-cost adsorbents. Desalination and Water Treatment, 12, 276–283.
Hydari, S., et al. (2012). A comparative investigation on removal performances of commercial activated carbon, chitosan biosorbent and chitosan/activated carbon composite for cadmium. Chemical Engineering Journal, 193–194, 276–282.
Khairul, M. A., Zanganeh, J., & Moghtaderi, B. (2019). The composition, recycling and utilisation of Bayer red mud. Resources, Conservation and Recycling, 141, 483–498.
Khajavian, M., et al. (2019). Simultaneous biosorption of nickel and cadmium by the brown algae Cystoseria indica characterized by isotherm and kinetic models. Applied Biological Chemistry, 62, 69.
Krishnani, K. K., et al. (2008). Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. Journal of Hazardous Materials, 153, 1222–1234.
Kumar, P. S., et al. (2011). Adsorption behavior of nickel (II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chemical Engineering Journal, 167, 122–131.
Li, P., et al. (2019). Recover iron from bauxite residue (Red Mud). IOP Conference Series: Earth and Environmental Science, 252, 042037.
Liao, B., et al. (2016). Equilibriums and kinetics studies for adsorption of Ni(II) ion on chitosan and its triethylenetetramine derivative. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 501, 32–41.
Liu, Z.-r, & Zhou, S.-q. (2010). Adsorption of copper and nickel on Na-bentonite. Process Safety and Environmental Protection, 88, 62–66.
Luu, T.-T., et al. (2022). Pb(II) adsorption mechanism and capability from aqueous solution using red mud modified by chitosan. Chemosphere, 287, 132279.
Lyklema, J. (1984). Points of zero charge in the presence of specific adsorption. Journal of Colloid and Interface Science, 99, 109–117.
Lyu, F., et al. (2021). Efficient removal of Pb(II) ions from aqueous solution by modified red mud. Journal of Hazardous Materials, 406, 124678.
Malkoc, E., & Nuhoglu, Y. (2005). Investigations of nickel (II) removal from aqueous solutions using tea factory waste. Journal of Hazardous Materials, 127, 120–128.
Matusiak, J., et al. (2022). The journey of tuning chitosan properties in colloidal systems: Interactions with surfactants in the bulk and on the alumina surface. Chemical Engineering Journal, 450, 138145.
Mondal, N. K., et al. (2017). Optimization of Cr (VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: Equilibrium, kinetic, thermodynamic and regeneration studies. Journal of Genetic Engineering and Biotechnology, 15, 151–160.
Ni, F., et al. (2015). Preparation and characterization of a cost-effective red mud/polyaluminum chloride composite coagulant for enhanced phosphate removal from aqueous solutions. Journal of Water Process Engineering, 6, 158–165.
Pap, S., et al. (2020). Synthesis optimisation and characterisation of chitosan-calcite adsorbent from fishery-food waste for phosphorus removal. Environmental Science and Pollution Research, 27, 9790–9802.
Sahu, M. K., et al. (2013). Removal of Pb(II) from aqueous solution by acid activated red mud. Journal of Environmental Chemical Engineering, 1, 1315–1324.
Shukla, S. R., & Pai, R. S. (2005). Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Separation and Purification Technology, 43, 1–8.
Smičiklas, I., et al. (2013). The influence of citrate anion on Ni(II) removal by raw red mud from aluminum industry. Chemical Engineering Journal, 214, 327–335.
Smičiklas, I., et al. (2014). Effect of acid treatment on red mud properties with implications on Ni(II) sorption and stability. Chemical Engineering Journal, 242, 27–35.
Smiljanić, S., et al. (2010). Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions. Chemical Engineering Journal, 162, 75–83.
Supanchaiyamat, N., et al. (2019). Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresource Technology, 272, 570–581.
Taneez, M., & Hurel, C. (2019). A review on the potential uses of red mud as amendment for pollution control in environmental media. Environmental Science and Pollution Research, 26, 22106–22125.
Thevannan, A., Mungroo, R., & Niu, C. H. (2010). Biosorption of nickel with barley straw. Bioresource Technology, 101, 1776–1780.
Vakili, M., et al. (2019). Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Separation and Purification Technology, 224, 373–387.
Wang, M., & Liu, X. (2021). Applications of red mud as an environmental remediation material: A review. Journal of Hazardous Materials, 408, 124420.
Wang, J., & Zhuang, S. (2022). Chitosan-based materials: Preparation, modification and application. Journal of Cleaner Production, 355, 131825.
Wang, L., et al. (2019). Application of red mud in wastewater treatment. Minerals, 9, 281.
Yang, S., et al. (2016). Mono/competitive adsorption of Arsenic(III) and Nickel(II) using modified green tea waste. Journal of the Taiwan Institute of Chemical Engineers, 60, 213–221.
Yang, T., et al. (2020). Enhancing Cd(II) sorption by red mud with heat treatment: Performance and mechanisms of sorption. Journal of Environmental Management, 255, 109866.
Zhang, L., et al. (2016). Crosslinked quaternized chitosan/bentonite composite for the removal of Amino black 10B from aqueous solutions. International Journal of Biological Macromolecules, 93, 217–225.
Zhou, X., & Zhou, X. (2014). The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chemical Engineering Communications, 201, 1459–1467.
Zhu, W., et al. (2017). Investigating the heavy metal adsorption of mesoporous silica materials prepared by microwave synthesis. Nanoscale Research Letters, 12, 323.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luu, TT., Nguyen, DK., Nguyen, T.T.P. et al. The effective Ni(II) removal of red mud modified chitosan from aqueous solution. Environ Monit Assess 195, 254 (2023). https://doi.org/10.1007/s10661-022-10877-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10877-0