Abstract
In this study, red mud modified by manganese dioxide(MRM) was utilized as an adsorbent to effectively remove Cd2+ from aqueous solution. The characteristics were analysed by SEM–EDS, XRD, BET, FTIR and XPS. Different factors that affected the Cd2+ removal on MRM, such as dosage, initial pH, initial Cd2+ concentration, were investigated using batch adsorption experiments. Simultaneously, the adsorption kinetics, adsorption isotherms and adsorption thermodynamics of Cd2+ were also investigated using adsorption experiments data. The characterization results showed that MRM had a rougher, larger specific surface area and pore volume (38.91 m2 g−1, 0.02 cm3 g−1) than RM (10.22 m2 g−1, 0.73 cm3 g−1). The adsorption experiments found that the equilibrium adsorption capacity of MRM for Cd2+ was significantly increased to 46.36 mg g−1, which was almost three times that of RM. According to the fitting results, the pseudo-second-order kinetic model described the adsorption process better than the pseudo-first-order kinetic model. The Langmuir model fitted the adsorption isotherms well, indicating that the adsorption process was unimolecular layer adsorption and the maximum capacity was 103.59 mg g−1. The thermodynamic parameters indicated that the adsorption process was heat-trapping and spontaneous. Finally, combined XPS and FTIR studies, it was speculated that the adsorption mechanisms should be electrostatic attachment, specific adsorption (i.e., Cd–O or hydroxyl binding) and ion exchange. Therefore, manganese dioxide modified red mud can be an effective and economical alternative to the removal of Cd2+ in the wastewater treatment process.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Heavy metal ions are extremely toxic and carcinogenic, seriously endangering the safety of water in today's world1. Cadmium (Cd) is one of the most toxic elements for humans, which is widely used in various industrial activities, inculding alloy manufacturing, smelting, electroplating, pigments, plastics and battery manufacturing2. Cd2+ could enter the human body through the food chain and deposit in various tissues of the body, which would lead to serious diseases and endanger human health and life3. According to the joint regulations of the World Health Organization (WHO), the U.S. Environmental Protection Agency (EPA) and the European Public Health Alliance (EPHA), the maximum amount of Cd2+ contamination in drinking water should not exceed 0.003 mg L−14. Currently, the main technologies for Cd2+ removal include chemical precipitation, membrane filtration, adsorption, ion exchange, electrodialysis, and biological methods. Among these treatment methods, adsorption has proved to be an effective and economical method for the removal of Cd2+ from the aqueous environment because of its simplicity, ease of operation, low cost, and excellent adsorption properties5. The adsorption method is mainly used to remove heavy metals by adsorption through the physicochemical action of heavy metal ions by the internal porous structure and surface active sites of the adsorbent material. Some adsorbents can be regenerated and recycled through a suitable desorption process. After years of research, researchers have developed a variety of adsorbent materials with strong adsorption capacity for heavy metals6,7. However, the practical application of most adsorbent materials is very limited due to the cost and time consuming limitations. Therefore, the preparation of low-cost and efficient heavy metal adsorbents remains a research priority in the field of wastewater treatment.
Over the past few years, red mud (RM), a solid waste generated during the production of the alumina industry, has received several attentions due to its huge pile and resource utilization difficulties8,9,10,11. Red mud is strongly alkaline, has large porosity and high dispersibility, which make it have a good adsorption effect on metal cations12. Simultaneously, its application as an adsorbent in the field of heavy metal wastewater treatment can realise both environmental and economic benefits13. However, the final removal effect could not reach the specified concentration limit due to the low adsorption capacity and strength structure of red mud. To further enhance the adsorption performance of red mud, scholars have adopted different activation methods, such as acidification14,15, heating16, soaking in sea water17. For example, some researchers have adopted simple acid activation and then precipitated red mud with ammonia to explore the possibility and adsorption rate of Cd2+ removal in water18. In addition, it is found that the adsorption stability of red mud for Cd2+ treated at 500 °C is almost twice that of the original red mud via studying the effect of heat treatment temperature on the adsorption capacity of Red mud for Cd2+16. However, the aforementioned modification methods are either costly to modify or have other problems, such as consuming too much thermal energy, difficult to perform, or low adsorption efficiency. One of the solutions to these problems is the use of metal compounds to modify the structure and surface area of the red mud, thus enhancing the adsorption properties.
Manganese dioxide (MnO2), an environmentally friendly metal oxide, has the advantages of large specific surface area, high surface activity, low price and easy availability19. More importantly, MnO2 has stable chemical properties and specific redox effects. MnO2 is an excellent adsorbent because of its ability to form complexes with heavy metal ions (e.g., Cd2+, Cu2+, Zn2+, and Pb2+) and good chemical stability under basic and acidic conditions20,21. For instance, amorphous hydrated manganese dioxide (HMO) had the selective removal of three metal ions (Pb2+, Cd2+, and Zn2+) and it was concluded that the adsorption of the three metal ions could be related to the inner sphere complexation20. Hydrated manganese dioxide had high efficient Cd2+ adsorption performance, and the maximum adsorption capacity for Cd2+ at 25 °C was 168.36 mg g−1, which was significantly higher than most heavy metal adsorption materials22. Unfortunately, the poor mechanical stiffness, excessive pressure drop and particular agglomeration limit the practical application of MnO2. MnO2 can be loaded on carriers with a large surface area to enhance adsorption and dispersibility23,24. Most materials with large surface areas are difficult and costly to fabricate, making them hard to scale up and apply. It is crucial to find low-cost carrier materials for MnO2, and bulk industrial solid waste, red mud, demonstrates its advantages in this regard.
The purpose of this study is to explore the behavior and mechanism of using amorphous MnO2 modified red mud to enhance the removal of cadmium. The manganese dioxide modified red mud (MRM) was prepared by the redox reaction between potassium permanganate and manganese sulfate for Cd2+ adsorption. The load level MnO2 could be controlled by changing the amount of red mud during the experiment. The introduced MnO2 is an amorphous phase, and the amorphous oxides have better adsorption properties than their crystals25. Then, it was investigated the effects of different factors on the adsorption of Cd2+. Besides, the adsorption kinetics and isotherms are discussed to explore the process of Cd2+ adsorption in detail and finally investigated the adsorption mechanism by Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS).
Results and discussion
Characterizations of adsorbents
XPS was used to characterise the RM and MRM surface elements. An increase in the intensity of the Mn 2p peak at 643.08 eV was observed in Fig. 1a, and the acceptable spectrum of Mn 2p is shown in Fig. 1b at 642.42 eV and 653.95 eV for Mn 2p3/2 and Mn 2p1/2, respectively. The binding energy difference between Mn 2p1/2 and Mn 2p3/2 is 11.54 eV, indicating that the Mn in MRM valence state is mainly Mn4+, which also indicates that the main form of manganese oxide on the load is MnO226,27.
The mineral composition of RM before and after loading was analysed by XRD, and the results are shown in Fig. 2a. The main phases of RM are minerals dominated by Si, Ca, and Al, such as Ca3Al2(SiO4)(OH)8(Katoite, Si-rich), Ca2SiO4(Calcium Silicate), Ca5(SiO4)2CO3(Spurrite) and Ca3Al2O6(Calcium Aluminum Oxide). After the loading of MnO2, the peak intensities of the characteristic peaks contained in RM all weakened and some even disappeared, but no new characteristic peaks were generated. This may be because the MnO2 loading of the amorphous phase weakened the intensity of the diffraction peaks of the other crystals28. It can be inferred that the MnO2 loaded on the surface of the red mud in this study is a non-chemo-metric amorphous phase, which is possibly due to the change in the crystalline structure of the manganese dioxide during the preparation of the material29.The FTIR spectra of RM and MRM at wavelengths of 400–4000 cm−1 were analysed and the results are shown in Fig. 2b. It can be seen from the figure that the diffraction peaks of each functional group changed significantly after the loading of MnO2 and new characteristic peaks appeared. A solid and broad absorption band appeared at 3432.58 and 3405.43 cm−1 for RM and MRM, respectively, which corresponds to the O–H stretching vibration of the hydroxyl group on the surface of the samples30,31. The band of MRM at 1635.13 cm−1 is attributed to the O–H stretching vibrations in Mn-OH20,32. The band at 1418.62 cm−1 appearing in RM is attributed to CO32+,proving that RM contains many carbonate compounds33,34. However, the CO32+peak in the MRM shifts and decreases in intensity after loading MnO2. The peaks appearing at 996.62 and 992.96 cm−1 are caused by O–Si–O stretching vibrations35,36. The presence of Al–O stretching vibrations (684.62 cm−1), Si–O–Al stretching vibrations (620.98 cm−1) and Fe–O stretching vibration (458.98 cm−1) existing in RM disappeared in the MRM37, while a clear absorption peak at 532.56 cm−1 appeared in the MRM. This band at 532.56 cm−1 indicates the presence of Mn–O stretching vibratio26, further verifying the successful loading of MnO2. In conclusion, MRM has rich surface functional groups, which makes MRM have vigorous chemisorption activity.
The SEM micrographs of RM and MRM are shown in Fig. 3. It can be seen that the RM (Fig. 3a) surface shows the irregular distribution and the presence of large particle agglomerates and lamellar particles with many irregular small particles in their gaps. After MnO2 loading (Fig. 3b), MRM was obviously covered by MnO2. Its surface was covered by many spherical, and flocculent particles appeared, showing a three-dimensional porous structure, which in turn provided more adsorption sites on the surface, pore channels and interlayer domains, thus improving the adsorption performance of MRM. Comparing the EDS energy spectra before and after modification (Fig. 4a,b), the content of manganese in the original red mud was not detected, but the content of main metal elements in the red mud changed after MnO2 loading. The increase of Mn content also indicated that the red mud had been successfully loaded with MnO2.
Porosity and pore size distribution of RM and MRM were determined by nitrogen adsorption–desorption experiments, and the results are shown in Fig. 5. According to the standard of International Union of Pure and Applied Chemistry (IUPAC), the adsorption–desorption isothermal curves of both RM and MRM are of type VI (Fig. 5a). Both showed obvious hysteresis back loops due to capillary condensation, indicating the existence of mesoporous structures inside. Meanwhile, the closed hysteresis loops are similar to the H3-type hysteresis loops, indicating that the pore structure is very irregular. The specific surface area and pore size were calculated based on the BET model and the BJH model, respectively, and the results are shown in Table 1. The apparent increase in the specific surface area of MRM is due to the formation of manganese oxides on its surface, which is consistent with the successful loading of MnO2 as shown in the SEM and EDS energy spectra. However, the average pore size of MRM is smaller than that of RM, which is since the loading of MnO2 changes RM from dispersed particles to more dense particles and the number of pores increases. The pore size distribution curves (Fig. 5b) also show that the pore size distribution of MRM is broader and the number of pores increases. In addition, the increase in pore capacity and the number of pores facilitates the diffusion of metal ions into the interior of the adsorbent, which is also one of the advantages of MRM.
Adsorption study
Effect of different ratios of manganese dioxide to red mud
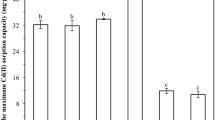
From Fig. 6, it was found that the different ratios of MnO2 and red mud had essential effects on the adsorption performance of MRM. The equilibrium adsorption capacity of the original red mud for Cd2+ was 18.73 mg g−1; when the ratio of manganese dioxide to red mud was 1:4, the equilibrium adsorption capacity of the generated MRM for Cd2+ in solution reached the highest, 44.93 mg g−1, which was 26.20 mg g−1 higher than that of the original red mud for Cd2+; the equilibrium adsorption capacities of the other five ratios were also higher than that of the original red mud. The improved adsorption capacity of the modified red mud was attributed to the surface loading of MnO2. The loading of MnO2 on the red mud resulted in a higher surface activity, giving it a high adsorption affinity for the metal cation Cd2+. In addition, because the red mud material is a good carrier, MnO2 can be better loaded and dispersed on its surface, enhancing the composite's adsorption performance38. When the ratio of MnO2 to red mud was 1:1, the adsorption effect was not as good as the modified red mud with a ratio of 1:4. This may be due to the deposition of excess MnO2 on the surface of the red mud, which leads to pore blockage and thus affects the adsorption effect39. Therefore, the modified red mud with a 1:4 ratio of MnO2 to red mud was chosen for the following experiments.
Effect of the adsorbent dosage
The adsorbent dosage is one of the critical parameters to determine the adsorption capacity of the material at a fixed initial concentration. The adsorption capacity and removal efficiency curves of Cd2+ on MRM are shown in Fig. 7. It can be seen from the figure that the removal efficiency of Cd2+ showed an apparent increasing trend in the range of 0.2–4 g L−1 with the increase of the MRM dosage. This is because the adsorption sites available for MRM increased with the increase of the dosage, which led to the increase of the removal efficiency. When the dosage exceeded 2 g L−1, the removal efficiency of Cd2+ increased slowly and finally reached equilibrium. At the same time, the adsorption capacity of MRM decreased with the increase of adsorbent dosage. In general, when the action of adsorbent and heavy metal ions reached equilibrium, the removal rate no longer increased. Continuing to increase the dosage of adsorbent will reduce the adsorption efficiency of adsorbent per unit mass. Therefore, to save materials and consider the adsorption capacity and removal efficiency, the optimal dosage of adsorbent was determined to be 1 g L−1 for further experiments.
Effect of initial pH
The pH value is one of the most critical parameters to control the heavy metal adsorption process because it can influence the surface properties of the adsorbent and the ionic species in the solution. The effect of pH on the adsorption performance of MRM was analysed by varying the pH (2–8) and the results are shown in Fig. 8. The initial pH has a significant effect on the adsorption effect of Cd2+, and the general rule is that the adsorption rate increases with the increase of pH value. The removal rate of Cd2+ increased from 21.18 to 86.70% when the pH was varied in the range of 2 to 6, and even the removal rate was close to 100% at pH 7 and 8.
At lower pH (2–4), the presence of a higher concentration of H+ in the solution caused the accumulation of a large amount of H+ around MRM, which in turn led to a positive charge of manganese hydroxyl functional group due to protonation, as shown by the reaction equation in Eq. (1)40. The electrostatic repulsion was produced between free Cd2+ in solution and positively charged MRM41. At the same time, high concentrations of H+ competed with Cd2+ for adsorption, resulting in a relatively low amount of adsorbed Cd2+. While at higher pH (4–6), there was less H+ in the solution and the competition for adsorption was weaker. And the manganese hydroxyl functional group underwent deprotonation and became negatively charged (Eq. (2)), generating electrostatic repulsion with Cd2+. The resulting result is an increase in MRM adsorption efficiency. When pH > 7.4, Cd2+ starts to produce precipitation, so the effect of pH on the adsorption performance of MRM under alkaline conditions is not discussed in this study.
The analysis of the Zeta potential of MRM at different pH conditions is shown in Fig. 9. It can be seen from the figure that before adsorption, the charge of MRM gradually increases with the increase of solution pH. The pH corresponding to when the zeta potential is zero is the isoelectric point of MRM (pHpzc = 2.2). After adsorption, the zeta potential of MRM decreases with the increase of pH and gradually stabilizes after pH > 4. From this, we can infer that Cd2+ can be adsorbed and neutralize the negative charge on the MRM surface or form metal complexes42.
Effect of coexisting cations
The simulated test wastewater in this study does not contain other ions, while metal cations such as Na+, K+, Ca2+ and Mg2+ are very common in natural waters or actual wastewater and easily exhibit competitive adsorption with the target heavy metal ions41. The effects of different competing ions such as Na+, K+, Ca2+ and Mg2+ on Cd2+ removal were investigated at an initial Cd2+ concentration of 50 mg L−1 and a coexisting ion concentration of 0.2 mmol L−1. The effect of coexisting ions on the adsorption of Cd2+ by MRM is shown in Fig. 10. The results showed that Na+, K+, Ca2+ and Mg2+ in the solution all decreased the adsorption of Cd2+ by MRM. This is because the presence of these cations in solution competes with Cd2+ for the limited adsorption sites on the surface of MRM, thus inhibiting the adsorption of Cd2+ on MRM.
Compared with the solution without the addition of any coexisting cations, the inhibition magnitude of Cd2+ adsorption by the same concentration of cations was in the order of Ca2+ > Mg2+ > K+ > Na+, with Ca2+ having the most substantial inhibition effect. This is due to the similar structure of Ca2+ and Cd2+. The relatively small hydrated ionic radius of Ca2+ makes it more easily to compete with Cd2+ for adsorption sites, and Ca2+ ions can rapidly bind to the active functional groups on the MRM43,44. More competing ions and impurities may exist in actual industrial wastewater and natural water bodies, such as many fibres, inorganic salts and pigments in paper industry wastewater. Therefore, the practical application of MRM requires further research on its removal effect on different types of wastewater.
Effect of the contact time and kinetics
Samples were taken for Cd2+ concentration at contact times of 10, 20, 30, 60, 90, 120, 150, 180, 240, 300, 360, 420, 480 min. The effects of contact time on the adsorption performance of MRM and RM are shown in Fig. 11a. The adsorption capacity of both RM and MRM for Cd2+ increased with time, but the adsorption capacity of MRM was always more significant than that of RM. RM reached saturation at the contact time of 60 min, and the adsorption capacity was maintained at 18 mg g−1 or less. The adsorption process of Cd2+ on MRM can be divided into two stages: the time at 0–180 min is the surface rapid adsorption stage, and the adsorption capacity of MRM increases to 44.72 mg g−1; after contact time more than 180 min, the adsorption of MRM on Cd2+ entered the slow adsorption stage, the adsorption capacity maintained at about 45 mg g−1, indicating that the adsorption equilibrium has been reached. This is because, at the early stage of adsorption, there are more free adsorption sites on the surface of MRM, the concentration gradient is large, and the adsorption rate is fast. However, with the increase of contact time, the number of free adsorption sites on the surface of MRM gradually becomes less, and the Cd2+ already adsorbed on MRM diffuses into the interior of the material along with the pore size, leading to the increasing diffusion resistance and the decrease of adsorption efficiency, and finally reaches the adsorption equilibrium.
The adsorption kinetic model is one of the essential tools to study the adsorption mechanism. The adsorption mechanism of the adsorbent is explored by studying the rate of solute adsorption by the adsorbent and fitting the data using a kinetic model. This study used pseudo-first-order, pseudo-second-order, external diffusion kinetic, and intraparticle diffusion models to fit the obtained adsorption data. The specific equations are as follows. The correlation coefficient was used to determine the fitting effect, and the fitting results are shown in Table 2.
The pseudo-first-order kinetic model:
The pseudo-second-order kinetic model:
The external diffusion kinetic model:
The intraparticle diffusion model:
where qt (mg g−1) is the amount of adsorption on the adsorbent at time t, qe (mg g−1) is the equilibrium Cd2+ adsorption capacity, k1 (g mg−1 min−1) and k2 (g mg−1 min−1) are the rate constants of pseudo-first-order adsorption and pseudo-second-order adsorption, respectively, c0 is the initial Cd2+ concentration, ct is the Cd2+ concentration at time t, kext (1/min) is the constant of external diffusion, kp (mg g−1 min1/2) is the intraparticle diffusion rate constant. The fitting curves obtained are shown in Fig. 11a–f.
From Table 2, the fit coefficients obtained from .the pseudo-first-order kinetic model and pseudo-second-order kinetic model were 0.9733 and 0.9904, respectively. The pseudo-second-order kinetic model was more suitable to describe the adsorption process of MRM, indicating that the adsorption process was dominated by chemisorption. This chemisorption involved electron sharing or electron transfer between the adsorbate and the adsorbent. The model fit of RM showed that the pseudo-first-order kinetic model was more consistent with the adsorption process of Cd2+ by RM, indicating that the adsorption process was physical adsorption. Applying the external diffusion model, we obtained the curves as Fig. 11c,d, and lnct showed a linear relationship with t in the first 180 min with R2 of 0.93, indicating that external diffusion was the dominant process in the initial adsorption process.
Thus, the intraparticle diffusion model analysed the rate-limiting phase on the Cd2+ adsorption process. According to Fig. 11e,f, qt is linear for t0.5, but the straight line does not pass through the origin, indicating that intraparticle diffusion is not the only rate-limiting step, and there may be other steps involved in controlling the adsorption rate. The fitting curves were divided into three linear parts possessing their respective rate constants, which followed the order kp,1 > kp,2 > kp,3. The first linearity represents the film diffusion process with the most considerable adsorption rate. This is due to the initial adsorption process in which the vacant adsorption sites on the MRM surface can rapidly bind to Cd2+. The second linearity is due to the intraparticle diffusion process. In this process, the surface adsorption sites gradually decrease, and the adsorption capacity starts to approach the maximum. Moreover, the reduction of adsorption sites causes the adsorbed Cd2+ to enter the interior of the mesopores, generating a considerable mass transfer resistance and finally reaching adsorption equilibrium with the adsorption rate maintained at kp,345,46.
Combining Fig. 11 and Table 2, it can be seen that MRM has higher metal adsorption capacity than RM. MRM has more active sites, larger specific surface area and pore volume, which contributes to the sufficient contact between metal ions and MRM and effectively reduces the diffusion resistance of metal ions in solution. All these results indicate that the modification of biochar by MnO2 is necessary and practical.
Effect of the initial Cd2+ concentration and adsorption isotherm
The effect of the initial concentration of Cd2+ on the adsorption effect of MRM is shown in Fig. 12. As can be seen from the figure, when the initial concentration of Cd2+ was low (10–100 mg L−1), the adsorption capacity of Cd2+ increased rapidly with the increase of concentration, and the removal rate decreased continuously. This is because there are enough adsorption sites on the MRM surface for Cd2+ adsorption when the initial concentration of heavy metals is low. At the initial concentration of Cd2+ higher than 150 mg L−1, the adsorption capacity of Cd2+ was unchanged due to the limited effective adsorption sites, and the maximum adsorption capacity of Cd2+ is 97.85 mg g−1.
The adsorption patterns of MRM on Cd2+ were investigated at three temperatures of 25 °C, 45 °C and 65 °C, as shown in Fig. 13. The common isothermal adsorption models are mainly Langmuir model and Freundich model, which were fitted to the data, and the fitted parameters are shown in Table 3.
The Langmuir model:
where qe (mg g−1) is the equilibrium Cd2+ adsorption capacity, qm (mg g−1) is the maximum Cd2+ adsorption capacity, ce is the equilibrium Cd2+ concentration of the solution, and KL (L mg−1) is the Langmuir adsorption constant. The essential characteristics of the Langmuir isotherm can be expressed in terms of the dimensionless constant separation factor or equilibrium parameter RL, which is expressed as follows47.
where c0 is the initial Cd2+ concentration and KL (L mg−1) is the Langmuir adsorption constant. For unfavorable adsorption, RL > 1; when RL = 1, linear adsorption happens; RL = 0 describe irreversible adsorption; favorable adsorption occurs while 0 < RL < 114.
The Freundlich model:
where qe (mg g−1) is the equilibrium Cd2+ adsorption capacity, ce is the equilibrium Cd2+ concentration of the solution, n is the Freundlich exponent, and KF (mg 1−1/n L1/n g−1) is Freundlich adsorption constant. 1/n value indicates the strength of the effect of concentration on adsorption, with 1 < n < 10 indicating favourable adsorption35,48.
From the fitted parameters in the Table 3, it can be seen that the Langmuir model better reflected the isotherms of the three tesmperatures than the Freundlich model, It indicated that the adsorption of MRM on Cd2+ was a monomolecular layer adsorption process, and the adsorption sites on the surface of MRM were uniformly distributed. The theoretical maximum adsorption amounts obtained by the fitting were 103.5857, 116.0454 and 118.4734 mg g−1, similar to the experimental results. The calculations showed that the separation coefficients RL in the Langmuir model were all in the range of 0 to 1, indicating that MRM was favourable for Cd2+ adsorption.
Adsorption thermodynamics
To investigate the thermodynamic behavior of Cd2+ adsorption on MRM, the adsorption process of MRM on Cd2+ was studied at three temperatures of 25 °C, 45 °C and 65 °C. The thermodynamic calculations of adsorption were performed using the temperature-dependent adsorption equation and Van't Hoff equation40.
The Gibbs free energy (ΔG) of the adsorption reaction was calculated with the following expression.
where qe (mg g−1) is the equilibrium Cd2+ adsorption capacity, ce is the equilibrium Cd2+ concentration of the solution, R (8.314 J mol−1 K−1) is the universal gas constant, T (K) is the absolute temperature and Kf (L g−1) is the distribution coefficient.
The enthalpy change of adsorption (ΔH) and the entropy change of adsorption (ΔS) are calculated as follows:
The relevant thermodynamic parameters ΔG, ΔH and ΔS were calculated from the above expressions(Eqs. (10)–(12)) and Fig. 14. As shown in Table 4. ΔH values are negative, which indicates the adsorption of Cd2+ by MRM is a heat-taking process. The values of ΔG are always negative at different temperatures and concentrations, representing that Cd2+ removal is favourable and spontaneous. ΔS values are negative, indicating that the adsorption process has an increased degree of freedom between the solid–liquid interface, which may be related to the ion exchange process49. The positive values of ΔH and ΔS reveal that the adsorption is a heat absorption and entropy increase process, corresponding to the isotherm study.
Regeneration of MRM
The ideal and economical adsorbent should be recyclable and reusable. The variation pattern of the adsorption capacity of Cd2+ by MRM with the number of Cd2+ adsorption/desorption cycles was given in Fig. 15. The results showed that after five Cd2+ adsorption/desorption cycles, the adsorption amounts of Cd2+ by MRM samples were reduced by about 35%. Apparently, MRM had good Cd2+ adsorption stability and regeneration ability.
Adsorption mechanism
To further determine the adsorption mechanism of Cd2+ on MRM, FTIR and XPS were used to analyse the elemental composition and chemical morphology of MRM before and after the adsorption of Cd2+. As shown in Fig. 16, no new vibrational peaks appeared in the FTIR after Cd2+ adsorption, but each functional group's peak intensity and wavenumber changed. The vibrational peak of –OH was weakened and shifted from 3405.43 to 3406.37 cm−1, which indicated that the hydroxyl group in MRM was involved in the adsorption process. In addition, the adsorption of Cd2+ led to a significant weakening and displacement of the Mn–OH absorption peak intensity, which was due to the entry of Cd2+ into the MRM and the ion exchange with the protons on the O atom of the Mn–OH group20. Thus, the Mn-OH group played an essential role in Cd2+ adsorption mainly through ion exchange50, demonstrating that MRM has some ion exchange capacity. The Mn–O functional group is similarly displaced, which may be due to the formation of an inner complex between the metal ion and MRM, in which a Cd–O bond was formed.
As shown in Fig. 17a, after the adsorption of Cd2+, the absorption peak of Cd 3d appeared in the XPS spectrum, indicating that MRM successfully adsorbed Cd2+. By fitting, two peaks of Cd 3d5/2 and Cd 3d3/2 with characteristic binding energies of 405.23 eV and 411.97 eV, respectively, appeared in the XPS spectrum of Cd 3d (Fig. 17b). The appearance of the characteristic peak of Cd 3d5/2 indicated the formation of endogenous compounds of Cd (–OCdOH), CdCO3, or Cd(OH)251,52. As shown in Fig. 17c,d, it was found that the Mn 2p spectra before and after the adsorption of MRM were very similar, with the characteristic peak binding energies of 653.88 and 642.11 eV for Mn 2p3/2 and Mn 2p5/2, respectively, indicating that Mn (IV) was always present before and after the adsorption.
According to previous reports, the O 1s profile consists of three peaks corresponding to hydroxide oxygen (OH−), lattice oxygen (O2−), and oxygen and hydroxide in molecular water (i.e., physisorbed, chemisorbed, and structural H2O and water in poor electrical contact with the mineral surface)26,53. As can be seen from Fig. 17e, before adsorption of Cd2+, the O 1s spectrum had three peaks at binding energies 530.112, 532.03, and 533.06 eV, corresponding to metal oxides (M–O), hydroxyl-containing metals (M–OH), and oxygen in adsorbed H2O, respectively. The intensity of the hydroxyl peak was significantly higher than the remaining two oxygen forms, confirming the presence of many hydroxyl groups on the surface of the MRM sample. After adsorption of Cd2+ (Fig. 17f), the M–OH percentage content decreased from 55.47 to 36.85%, indicating that Cd2+ interacts with M–OH to form hydroxy complexes (hydroxy composites) or ion exchange. In contrast, the M–O percentage content increased from 37.74 to 49.89%, indicating the formation of Mn–O–R (R: functional group) or Cd–O may be formed on the surface of MRM after adsorption. In addition, because the reaction of MRM with Cd2+ consumed M–OH and increased Mn–O, this process formed H2O, thus increasing the percentage of H2O54. In summary, it can be concluded that the mechanism of cadmium adsorption by MRM may include ion exchange, internal sphere complexation and electrostatic attraction.
Comparison of MRM with other adsorbents for Cd2+ removal
To further evaluate the adsorption capacity of MRM, the Cd2+ removal effect of MRM and other commonly used heavy metal adsorbent materials were compared under the same experimental conditions(pH = 6, T = 25 °C, dosage = 1 g L−1, t = 240 min and initial Cd2+ concentration = 50 mg L−1.), and the obtained results are shown in Table 5. From Table 5, it can be seen that the removal of Cd2+ by MRM is better than many adsorbent materials reported in the literature, so MRM can be used for medium heavy metal removal in aqueous solutions, and since RM is an industrial waste and MnO2 is a cheap metal oxide, MRM can be prepared at a small cost.
Leaching metals of MRM
The leaching metal concentrations obtained from the MRM leaching test were shown in Table 6, from which we can see that the leaching toxicity of the red mud itself was weaker, and the leaching toxicity of the red mud modified by MnO2 was lower than the former. The MRM leaching metal concentrations were all lower than the limits of the standards for drinkingwater quality of China (GB5749-2006). Even the content of such major elements as Al and Ca was less than the standard mass value in GB 5749-2006. Therefore, the data of leached metals indicated the safety of MRM for use in aqueous environment.
Conclusions
In this study, the behavior and mechanism were conducted using amorphous MnO2 modified red mud to enhance the removal of cadmium, At the same time, the influencing factors of the adsorption capacity of Cd2+ in different process conditions were studied through adsorption experiments, including the adsorption kinetics, adsorption isotherm and adsorption thermodynamics of Cd2+.
Based on the findings of this study, the following conclusions and recommendations are drawn:
-
1.
MnO2-modified red mud effectively improved the specific surface area and pore capacity and good adsorption effect on Cd2+. The adsorption performance of MRM on Cd2+ was affected by the ratio of MnO2 and red mud, pH, dosage, initial concentration of Cd2+, coexisting ions and contact time.
-
2.
The pseudo-first-order kinetic model is more suitable than the pseudo-second-order kinetic model to describe the adsorption process of MRM, indicating that the chemisorption of Cd2+ on the surface of MRM occurs through electron sharing or electron transfer. The Langmuir model had a higher fitting coefficient than the Freundlich model, indicating that the adsorption of MRM on Cd2+ was a monomolecular layer adsorption process, and the surface adsorption sites are uniformly distributed. The fitted theoretical maximum adsorption amount is 103.5857 mg g−1, and the separation coefficient RL is in the range of 0 to 1, indicating that MRM was favourable for Cd2+ adsorption. The thermodynamic adsorption study indicated that the adsorption reaction was a spontaneous heat absorption process.
-
3.
Combined XPS and FTIR studies, the adsorption mechanism of MRM on Cd2+ was speculated to be that MRM relied on the ion exchange, internal sphere complexation and electrostatic attraction of its surface hydroxyl groups to achieve the adsorption of Cd2+.
-
4.
Using MnO2 to modify red mud not only provides a good method to recover red mud as a waste water treatment material, but also greatly improves the adsorption performance of the red mud, which can be used as an adsorbent to treat cadmium-containing wastewater.
Materials and methods
Sources of the RM and reagents
The original red mud was obtained from an aluminium plant in Chongqing (Chongqing Municipality, China), where industrial alumina refining is the combined process. The main chemical composition of red mud included: CaO (30.32%), SiO2 (21.42%), Fe2O3 (14.90% ), Al2O3 (10.96%), Na2O (7.03%), TiO2 (5.60%), MgO (0.58%), K2O (0.26%), with a burn vector of 6.4%.The raw materials were crushed and passed through a 100 mesh sieve. The sample was dried in an oven (DHG-9240A, Shenzhen) at 105 °C for 48 h, sealed and stored in plastic bags as experimental raw materials, and named RM.
Potassium permanganate (KMnO4), manganese sulfate monohydrate (MnSO4·H2O), cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O), nitric acid (HNO3), sodium hydroxide (NaOH), calcium chloride (CaCl2), magnesium chloride (MgCl2), potassium chloride (KCl) and sodium chloride (NaCl), are analytically pure and used without further purification. The water used for the experiments was deionised water.
Preparation of MRM
Manganese dioxide modified red mud was prepared by the redox precipitation method using KMnO4 and MnSO4·H2O as manganese sources. The detailed preparation procedures are as follows.
-
i.
We selected 0.01 mol as the initial quantity of reactants and accurately weighed 1.581 g KMnO4 and 1.690 g MnSO4·H2O ((KMnO4/MnSO4·H2O molar ratio = 1:1) and dissolved them in 100 mL of deionised water, respectively. A certain amount of red mud (MnO2:RM mass ratio = 1:1, 1:2, 1:3, 1:4, 1:5, 1:6) was added to a beaker containing 30 ml of deionised water to form a suspension of red mud. KMnO4 aqueous solution was added to the red mud suspension and stirred at 300 rpm in a constant temperature chamber for 0.5 h to make full contact between the two. Then MnSO4 solution was added to the above-mixed solution, and the dark brown precipitation of MnO2 in the suspension was produced according to Eq. (13).
$$\begin{array}{c}3{\mathrm{Mn}}^{2+}+3{\mathrm{MnO}}_{4}^{-}+2{\mathrm{H}}_{2} {\text{O}}=5\text{Mn}{\mathrm{O}}_{2}\downarrow +4{\mathrm{H}}^{+}\end{array}$$(13) -
ii.
After stirring at constant temperature for 0.5 h, the solution was placed in a constant temperature oscillator (35 °C, 200 rpm) for 2 h. The solution was aged for 24 h. After it cooled, it was washed and centrifuged (4000 rpm) several times to remove the residual KMnO4 and MnSO4, and finally dried at 60 °C for 8 h. The dried sample was calcined in a muffle furnace at 400 °C for 3 h to obtain the MRM sample. After cooling, it was ground into powder and stored in a container box for further experimental study. Preparation flow chart is as follows in Fig. 18.
Characterisation analysis
In this study, the crystal phase and composition of the sample was analysed by the X-ray diffractometer (XRD, RIGAKU, Japan), with the conditions of Cu Kα as the radiation, λ = 1.5406 Å, and scanning speed of 5° min−1. The scanning electron microscope (SEM) and energy-dispersive spectroscopic (EDS, JSM-7500F, Japan) were obtained to investigate surface morphology and elemental composition changes of the samples. The specific surface area of the samples was measured by N2 adsorption–desorption experiments at 200 °C with a degassing time of 6 h using a Mike 2460 physisorption instrument and calculated by the Brunauer–Emmett–Teller (BET). The pore size of the sample was measured and calculated by Barret-Joyner-Halender (BJH) method in the N2 adsorption–desorption experiments. Fourier transform infrared spectrometer (FTIR, Nicolet 670, USA) was used to measure the infrared spectra of the samples and analyse the functional groups contained in the samples. In addition, the samples' X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, USA) was made with Al Kα (hv = 1486.6 eV) rays as the excitation source corrected for C1s = 284.80 eV binding energy to investigate the adsorption mechanism further.
Batch adsorption and detection of Cd2+
Batch adsorption experiments investigated the adsorption performance of MRM on Cd2+. Cd(NO3)2·4H2O was used as the cadmium source, and 1.386 g of Cd(NO3)2·4H2O was weighed and dissolved in 500 ml of deionised water to produce 1000 mg L−1 of cadmium stock solution. In this study, the effects of different ratios of MnO2 and red mud, dosage, initial pH, initial Cd2+ concentration, coexisting ions, contact time and temperature on the adsorption performance of the materials were investigated sequentially. A certain amount of MRM was added to a 250 mL conical flask containing 100 mL Cd2+ solution and then shaken in a shaker bath (7HZ-82, Changzh·ou) for 4 h at 200 rpm. The temperature range was 25–65 °C, the dosage range was 0.02–1.0 g, the pH range was 2–8, the initial Cd2+ concentration range was 10–300 mg L−1, the contact time range was 10–480 min, and the coexisting Na+, K+, Ca2+ and Mg2+ were selected at a concentration of 0.2 mmol L−1. After oscillation, all samples were filtered through a 0.45 μm membrane, and the Cd2+ concentration in the solution was measured by atomic absorption spectrophotometer (AA 6100, Techcomp) at 228.8 nm. The removal rate and equilibrium adsorption capacity of Cd2+ were calculated according to Eqs. (14) and (15).
where E (%) is Cd2+ removal efficiency, c0 and ce (mg L−1) are initial and equilibrium Cd2+ concentration in the solution, m (g) is the mass of MRM, qe (mg g−1) is equilibrium Cd2+ adsorption capacity, and V(L) is solution volume.
Leaching metal test
To examine the risk of toxic leaching of the material, the Chinese standard determination procedure (HJ/T299-2007) was adopted for MRM. A mixture of concentrated sulfuric acid and concentrated nitric acid with a mass ratio of 2:1 was added to water (about 2 drops of the mixture in 1 L of water) to prepare a leaching agent with pH 3.20 ± 0.05. The components to be measured (red mud and MRM) and leaching agent were added at a liquid–solid ratio of 10:1 (kg L−1), shaken for 20 h at 120 rpm and 25 °C. The number of hazardous components leached from the supernatant was determined after centrifugation.
Desorption and regeneration studies
The MRM adsorbent was regenerated by five cycles (the adsorption test conditions were at pH = 6, T = 25 °C, dosage = 1 g L−1, t = 240 min and initial Cd2+ concentration = 50 mg L−1). The adsorbed MRM was adsorbed with 0.1 mol L−1 HCl to remove Cd2+, then washed three times with deionized water and dried to constant weight in a vacuum drying oven at 60 °C.
Data availability
All data included in this study are available from the corresponding author on reasonable request.
References
Zhan, S., Wu, Y., Wang, L., Zhan, X. & Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 86, 353–368 (2016).
Rao, K., Mohapatra, M., Anand, S. & Venkateswarlu, P. Review on cadmium removal from aqueous solutions. Int. J. Eng. Sci. Technol. 2, 81–103 (2011).
Esen, C., Şenay, R. H., Feyzioğlu, E. & Akgöl, S. Poly(hydroxyethyl methacrylate-co-methacryloylglutamic acid) nanospheres for adsorption of Cd2+ ions from aqueous solutions. J. Nanoparticle Res. 16, 1–11 (2014).
Siegel, F. R. Contaminant/natural background values: timing and processes. In Environmental Geochemistry of Potentially Toxic Metals 77–101 (2002).
Uddin, M. K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017).
Liang, J. et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem. Eng. J. 273, 101–110 (2015).
Dong, L., Zhu, Z., Ma, H., Qiu, Y. & Zhao, J. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin. J. Environ. Sci. 22, 225–229 (2010).
Wang, X. et al. Dealkalization of Red Mud by Carbide Slag and Flue Gas. Clean - Soil, Air, Water 46, 3–9 (2018).
Lyu, F., Hu, Y., Wang, L. & Sun, W. Dealkalization processes of bauxite residue: A comprehensive review. J. Hazard. Mater. 403, 123671 (2021).
Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2, 316–331 (2016).
Wang, L. et al. Application of red mud in wastewater treatment. Minerals 9, 281 (2019).
Kaixuan, H. U. Study on influence of pollutants in red mud yard on groundwater environment. (2019).
Bhatnagar, A., Vilar, V. J. P., Botelho, C. M. S. & Boaventura, R. A. R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 32, 231–249 (2011).
Tsamo, C., Djomou Djonga, P. N., Dangwang Dikdim, J. M. & Kamga, R. Kinetic and equilibrium studies of Cr(VI), Cu(II) and Pb(II) removal from aqueous solution using red mud, a low-cost adsorbent. Arab. J. Sci. Eng. 43, 2353–2368 (2018).
Almeida, A. C. M. et al. Chemically activated red mud: Assessing structural modifications and optimizing adsorption properties for hexavalent chromium. Colloids Surf. A Physicochem. Eng. Asp. 628, 127325 (2021).
Yang, T. et al. Enhancing Cd(II) sorption by red mud with heat treatment: Performance and mechanisms of sorption. J. Environ. Manag. 255, 109866 (2020).
Grudić, V. V. et al. Pb(II) and Cu(II) sorption from aqueous solutions using activated red mud—Evaluation of kinetic, equilibrium, and thermodynamic models. Polish J. Environ. Stud. 22, 377–385 (2013).
Sahu, M. K., Mandal, S., Yadav, L. S., Dash, S. S. & Patel, R. K. Equilibrium and kinetic studies of Cd(II) ion adsorption from aqueous solution by activated red mud. Desalin. Water Treat. 57, 14251–14265 (2016).
He, C. & Xie, F. Adsorption behavior of manganese dioxide towards heavy metal ions: Surface zeta potential effect. Water. Air. Soil Pollut. 229, 1–13 (2018).
Su, Q., Pan, B., Wan, S., Zhang, W. & Lv, L. Use of hydrous manganese dioxide as a potential sorbent for selective removal of lead, cadmium, and zinc ions from water. J. Colloid Interface Sci. 349, 607–612 (2010).
Zhang, H. et al. Removal of aqueous Pb(II) by adsorption on Al2O3-pillared layered MnO2. Appl. Surf. Sci. 406, 330–338 (2017).
Zhai, Y. & Wang, H. Kinetics and mechanism study on adsorption of cadmium by freshly synthesized hydrous manganese dioxide. Desalin. Water Treat. 57, 6981–6990 (2016).
Fayazi, M., Afzali, D., Ghanei-Motlagh, R. & Iraji, A. Synthesis of novel sepiolite–iron oxide–manganese dioxide nanocomposite and application for lead(II) removal from aqueous solutions. Environ. Sci. Pollut. Res. 26, 18893–18903 (2019).
Afzali, D. & Fayazi, M. Deposition of MnO2 nanoparticles on the magnetic halloysite nanotubes by hydrothermal method for lead(II) removal from aqueous solutions. J. Taiwan Inst. Chem. Eng. 63, 421–429 (2016).
Zhang, C. et al. Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chem. Eng. J. 284, 247–259 (2016).
Peng, L. et al. Manganese dioxide nanosheet suspension: A novel absorbent for Cadmium(II) contamination in waterbody. J. Colloid Interface Sci. 456, 108–115 (2015).
Wagner, C. D., Riggs, W. M., Davis, L. E. & Moulder, J. F. Handbook of X-ray Photoelectron Spectroscopy. (1979).
Escande, V., Petit, E., Garoux, L., Boulanger, C. & Grison, C. Switchable alkene epoxidation/oxidative cleavage with H2O2/NaHCO3: Efficient heterogeneous catalysis derived from biosourced Eco-Mn. ACS Sustain. Chem. Eng. 3, 2704–2715 (2015).
Wang, S. G. et al. Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Purif. Technol. 58, 17–23 (2007).
Lyu, F. et al. Efficient removal of Pb(II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 406, 124678 (2021).
Nejadshafiee, V. & Islami, M. R. Intelligent-activated carbon prepared from pistachio shells precursor for effective adsorption of heavy metals from industrial waste of copper mine. Environ. Sci. Pollut. Res. 27, 1625–1639 (2020).
Pagnanelli, F., Sambenedetto, C., Furlani, G., Vegliò, F. & Toro, L. Preparation and characterisation of chemical manganese dioxide: Effect of the operating conditions. J. Power Sources 166, 567–577 (2007).
Liu, J. et al. Novel red mud/polyacrylic composites synthesized from red mud and its performance on cadmium removal from aqueous solution. J. Chem. Technol. Biotechnol. 95, 213–222 (2020).
Cao, J. L. et al. Mesoporous modified-red-mud supported Ni catalysts for ammonia decomposition to hydrogen. Int. J. Hydrogen Energy 39, 5747–5755 (2014).
Lakshmi Narayanan, S., Venkatesan, G. & Vetha Potheher, I. Equilibrium studies on removal of lead (II) ions from aqueous solution by adsorption using modified red mud. Int. J. Environ. Sci. Technol. 15, 1687–1698 (2018).
Khan, T. A., Chaudhry, S. A. & Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 202, 165–175 (2015).
Smičiklas, I., Smiljanić, S., Perić-Grujić, A., Šljivić-Ivanović, M. & Antonović, D. The influence of citrate anion on Ni(II) removal by raw red mud from aluminum industry. Chem. Eng. J. 214, 327–335 (2013).
Liang, J. et al. Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 5, 5049–5058 (2017).
Zhang, H. et al. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. Sci. Rep. 10, 1–13 (2020).
Gheju, M., Balcu, I. & Mosoarca, G. Removal of Cr(VI) from aqueous solutions by adsorption on MnO2. J. Hazard. Mater. 310, 270–277 (2016).
Su, Q. et al. Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci. Total Environ. 407, 5471–5477 (2009).
Wang, Z. et al. Adsorption of uranium(VI) to manganese oxides: X-ray absorption spectroscopy and surface complexation modeling. Environ. Sci. Technol. 47, 850–858 (2013).
Huang, X., Hao, H., Zhang, G., Li, J. & Ji, P. Adsorption of Cd2+ from wastewater by modified fly ash. Chin. J. Appl. Ecol. 30, 3215–3223 (2019).
Ji, H. et al. Cadmium adsorption by biochar prepared from pyrolysis of silk waste at different temperatures. Chin. J. Appl. Ecol. 29, 1328–1338 (2018).
Wu, S., Xie, F., Chen, S. & Fu, B. The removal of Pb (II) and Cd (II) with hydrous manganese dioxide: Mechanism on zeta potential and adsorption behavior. Environ. Technol. 41, 3219–3232 (2020).
Wang, W. Equilibrium, kinetics and thermodynamics study on the adsorption of Cr(VI) and as(III) by diatomite-modified MnO2. J. Dispers. Sci. Technol. 0, 1–14 (2020).
Namasivayam, C. & Senthilkumar, S. Removal of arsenic (V) from aqueous solution using industrial solid waste: Adsorption rates and equilibrium studies. Ind. Eng. Chem. Res. 37, 4816–4822 (1998).
Nethaji, S. & Sivasamy, A. Removal of hexavalent chromium from aqueous solution using activated carbon prepared from walnut shell biomass through alkali impregnation processes. Clean Technol. Environ. Policy 16, 361–368 (2014).
Xiyili, H., Çetintaş, S. & Bingöl, D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics. Process Saf. Environ. Prot. 109, 288–300 (2017).
Sari, A. & Tuzen, M. Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Appl. Clay Sci. 88–89, 63–72 (2014).
Tkachenko, O. P., Shpiro, E. S., Wark, M., Schulz-Ekloff, G. & Jaeger, N. I. X-ray photoelectron/X-ray excited auger electron spectroscopic study of highly dispersed semiconductor CdS and CdO species in zeolites. J. Chem. Soc. Faraday Trans. 89, 3987–3994 (1993).
Zhang, F. et al. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 153, 68–73 (2015).
Wang, Y., Feng, X., Villalobos, M., Tan, W. & Liu, F. Sorption behavior of heavy metals on birnessite: Relationship with its Mn average oxidation state and implications for types of sorption sites. Chem. Geol. 292–293, 25–34 (2012).
Meng, K. et al. Efficient adsorption of the Cd(II) and As(V) using novel adsorbent ferrihydrite/manganese dioxide composites. ACS Omega 4, 18627–18636 (2019).
Acknowledgements
This research was financially supported by the National Key R&D Program of China (No. 2019YFC1905600).
Author information
Authors and Affiliations
Contributions
Y.P.: carried out the experiments and drafted the paper. C.Z.: analyzed the data. Y.L. and X.B.: contributed reagents/materials/analysis tools. Q.L. and T.H.: contributed on the writing of the manuscript. D.P.P.: reviewed and revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pang, Y., Zhao, C., Li, Y. et al. Cadmium adsorption performance and mechanism from aqueous solution using red mud modified with amorphous MnO2. Sci Rep 12, 4424 (2022). https://doi.org/10.1038/s41598-022-08451-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08451-2
- Springer Nature Limited
This article is cited by
-
The Study of Acid Leaching on the Mineralogical and Microscopic Changes of Red Mud
Mining, Metallurgy & Exploration (2024)
-
Adsorptive removal of potentially toxic Pb2+ from aqueous medium using functionalized Pycnanthus angolensis seed as a precursor
Biomass Conversion and Biorefinery (2024)
-
Preparation, characterization and application of the Pb-ion-imprinted polymers based on epoxy resin and polyamide-amine dendrimers
Polymer Bulletin (2024)
-
Mechanism of Deep-Sea Microbial-Mediated Synergistic Synthesis of Ferromanganese Oxidation Products Process for Heavy Metal Ion Adsorption
JOM (2023)
-
Adsorption mechanism and modeling of radionuclides and heavy metals onto ZnO nanoparticles: a review
Applied Water Science (2023)