Abstract
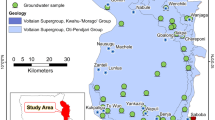
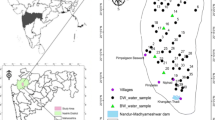
Dawa River basin in southern Ethiopia is covered by volcanic, basement, and sedimentary rocks. Locating good quality groundwater is a challenge in most parts of the basin. Statistical analysis and graphical plots of 94 hydrochemical data of groundwater were used as a main tool to acquire an insight into the major processes that control groundwater chemistry. In the volcanic terrain groundwater is dilute (mean total dissolved solids (TDS): 152 mg/l), while salinity is the highest in the sedimentary terrain (mean TDS: 1750 mg/l). NO3 − varies from below the detection limit to 433 mg/l NO3 −. In 26 % of the water samples, nitrate concentration is above the human-affected value, 5 mg/l NO3 −. In 6 % of the samples, NO3 − concentration is above the limit recommended in drinking water, 50 mg/l NO3 −, by WHO. Concentration range of the other major ions is also high and hydrochemical water types are diverse, suggesting the effect of various hydrogeochemical processes on the water chemistry. Chemical data analysis revealed that in the volcanic and most parts of the basement terrains silicate hydrolysis is the dominant process. Gypsum dissolution is the main process in the sedimentary terrain. Dissolution of gypsum is also important at few locations along dry riverbeds in the semiarid area where the effect of evaporation on the water chemistry is considerable. Loading of factors with K+ and SO4 2−, K+ and NO3 −, and NO3 − and correlation of SO4 2− with Cl−, along with the observed high nitrate concentration, indicate the effect of surface contamination sources on the water quality.








Similar content being viewed by others
References
Adams, S., Titus, R., Pietersen, K., Tredoux, G., & Harris, C. (2001). Hydrochemical characteristics of aquifers near Sutherland in the western Karoo, South Africa. Journal of Hydrology, 241(2001), 81–93. doi:10.1016/s0022-1694(00)00370-x.
Appelo, C. A. J., & Postma, D. (2005). Geochemistry, groundawater and pollution. Leiden: A.A. Balkema.
Assefa, G. (1988). Potential hydrocarbon-generating rock units within the phanerozoic sequence of the Ogaden Basin, Ethiopia: a preliminary assessment using the Lopatin model. Journal of Petroleum Geology, 11(4), 461–472. doi:10.1111/j.1747-5457.1988.tb00832.x.
Batayneh, A., & Zumlot, T. (2012). Multivariate statistical approach to geochemical methods in water quality factor identification: application to the shallow aquifer system of Yarmouk Basin of North Jordan. Research Journal of Environmental and Earth Sciences, 4(7), 756–768.
Biswas, A., Nath, B., Bhattacharya, P., Halder, D., Kundu, A. K., Mandal, U., Mukherjee, A., Chatterjee, D., Mӧrth, C., & Jacks, G. (2012). Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal Basin: consequences for sustainable drinking water supply. Science of the Total Environment, 431, 402–412. doi:10.1016/j.scitotenv.2012.05.031.
Cho, J. C., Cho, H. B., & Kim, S. J. (2000). Heavy contamination of a subsurface aquifer and a stream by livestock wastewater in a stock farming area, Wonju, Korea. Environmental Pollution, 109(1), 137–146. doi:10.1016/S0269-7491(99)00230-4.
Custodio, E. (2003). Groundwater in volcanic hard rocks. In J. Krásný & J. M. Sharp (Eds.), Groundwater in fractured rocks: selected papers from the groundwater in fractured rocks (Vol. 9, pp. 95–108). London: Taylor & Francis.
Datta, P. S., & Tyagi, S. K. (1996). Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. Journal of the Geological Society of India, 47, 179–188.
Davis, S. N., Whittemore, D. O., & Fabryka-Martin, J. (1998). Uses of chloride/bromide ratios in studies of potable water. Groundwater, 36(2), 338–350.
Deyassa, G., Kebede, S., Ayenew, T., & Kidane, T. (2014). Crystalline basement aquifers of Ethiopia: their genesis, classification, and aquifer properties. Journal of African Earth Sciences, 100(2014), 191–202. doi:10.1016/j.jafrearsci.2014.06.002.
Elisante, E., & Muzuka, A. N. N. (2015). Occurrence of nitrate in Tazanian groundwater aquifers: a review. Applied Water Science. doi:10.1007/s13201-015-0269-z.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater. New Jersey: Prentice-Hall.
Gat, J. R. (2010). Isotope hydrology: a study of the water cycle (series on environmental science and management (Vol. 6). London: Imperial college press.
Geleta S (1998) Biostratigraphy, depositional environment, basin evolution and hydrocarbon potential of the late Triassic to late Jurassic succession, Ogaden Basin, Ethiopia. Dissertation, Unversity of Tübingen.
Gamachu, D. (1977). Aspects of climate and water budget in Ethiopia. Addis Ababa: Addis Ababa University press.
Ghebreab, W. (1992). The geological evolution of the Adola Precambrian greenstone belt, southern Ethiopia. Journal of African Earth Sciences (and the Middle East), 14(4), 457–469. doi:10.1016/0899-5362(92)90078-Q.
Hamrla, M. (1977). The Adola gold field, Ethiopia: geology and genetic hypothesis. Geologija Letnik, 20, 247–282 http://www.dlib.si/?URN=URN:NBN:SI:DOC-YHUQZCE8. Accessed 02 June 2016.
Hem, JD (1985). Study and interpretation of the chemical characteristics of natural water (3rd ed), U.S Geological Survey Water Supply Paper 2254. Washington: US government printing office.
Hiscock, K. M. (2005). Hydrogeology: principles and practice. Malden: Blackwell.
Hunegnaw, A., Sage, L., & Gonnard, R. (1998). Hydrocarbon potential of the intracratonic Ogaden Basin, SE Ethiopia. Journal of Petroleum Geology, 21(4), 401–425. doi:10.1111/j.1747-5457.1998.tb00793.x.
IAEA (2013). Global network of isotopes in precipitation. The GNIP Database IAEA web. http://www.iaea.org/water. Accessed on 12 April 2013.
Jalali, M. (2006). Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamadan, Iran. Environmental Geology, 51, 433–446. doi:10.1007/s00254-006-0338-6.
Jalali, M. (2010). Groundwater geochemistry in the Alisadr, Hamadan, western Iran. Environmental Monitoring and Assessment, 166, 359–369. doi:10.1007/s10661-009-1007-5.
Kebede, S. (2013). Groundwater in Ethiopia: features, numbers, and opportunities. Berlin: Springer.
Keene, W. C., Pszenny, A. A. P., Galloway, J. N., & Hawley, M. E. (1986). Sea-salt corrections and interpretation of constituent ratios in marine precipitation. Journal of Geophysical Research-Atmospheres, 91, 6647–6658. doi:10.1029/JD091iD06p06647.
Kim, K., Rajmohan, N., Kim, H. J., Kim, S. H., Hwang, G. S., Yun, S. T., Gu, B., Cho, M. J., & Lee, S. H. (2005). Evaluation of geochemical processes affecting groundwater chemistry based on mass balance approach: a case study in Namwon, Korea. Geochemical Journal, 39, 357–369.
Leenhouts, J. M., Bassett, R. L., & Maddock III, T. (1998). Utilization of intrinsic boron isotopes as co-migrating tracers of identifying potential nitrate contamination sources. Groundwater, 36(2), 240–250. doi:10.1111/j.1745-6584.1998.tb01089.x.
Li, P., Qian, H., Wu, J., Zhang, Y., & Zhang, H. (2013). Major ion chemistry of shallow groundwater in the Dongsheng coalfield, Ordos Basin, China. Mine Water and the Environment, 32(3), 195–206. doi:10.1007/s10230-013-0234-8.
Lu, Y., Tang, C., Chen, J., & Chen, J. (2015). Groundwater recharge and hydrogeochemical evolution in Leizhou Peninsula, China. Journal of Chemistry. doi:10.1155/2015/427579 .Accessed on 03 June 2016
Mohr, P., & Zanettin, B. (1988). The Ethiopian flood basalt province. In J. D. Macdougall (Ed.), Continental flood basalts (pp. 63–110). Dordrecht: Kluwer Academic.
Mueller, D. K., Hamilton, P. A., Helsel, D. R., Hitt, K. J., & Ruddy, B. C. (1995). Nutrients in groundwater and surface water of the United States—an analysis of data through 1992 (water-resources investigation report 95–4031). Colorado: US Geological Survey.
Panno, S. V., Kelly, W. R., Martinsek, A. T., & Hackley, K. C. (2006). Estimating background and threshold nitrate concentrations using probability graphs. Groundwater, 44(5), 697–709. doi:10.1111/j.1745-6584.2006.00240.x.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analysis. Eos, Transactions of the American Geophysical Union, 25(6), 914–923. doi:10.1029/TR025i006p00914.
Postma, D., Boesen, C., Kristiansen, H., & Larsen, F. (1991). Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resources Research, 27(8), 2027–2045. doi:10.1029/91WR00989.
Rajmohan, N., & Elango, L. (2004). Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River basins, southern India. Journal of Environmental Earth Sciences, 46, 47–61. doi:10.1007/s00254-004-1012-5.
Sonkamble, S., Sahya, A., Mondal, N. C., & Harikumar, P. (2012). Appraisal and evolution of hydrochemical processes from proximity basalt and granite areas of Deccan volcanic province (DVP) in India. Journal of Hydrology, 438-439, 181–193. doi:10.1016/j.jhydrol.2012.03.022.
Stumm, W., & Morgan, J. J. (1995). Aquatic chemistry. New York: Wiley-Interscience.
Subramani, T., Rajmohan, N., & Elango, L. (2010). Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, southern India. Environmental Monitoring and Assessment, 162, 123–137. doi:10.1007/s10661-009-0781-4.
Thimonier, A., Schmitt, M., Waldner, P., & Schleppi, P. (2008). Seasonality of the Na/Cl ratio in precipitation and implications of canopy leaching in validating chemical analyses of through fall samples. Atmospheric Environment, 42(40), 9106–9117. doi:10.1016/j.atmosenv.2008.09.007.
Usunoff, E. J., & Guzmán-Guzmán, A. (1989). Multivariate analysis in hydrochemistry: an example of the use factor and correspondence analyses. Groundwater, 27(1), 27–34. doi:10.1111/j.1745-6584.1989.tb00004.x.
WHO (2008). Guidelines for drinking-water quality—volume 1: recommendations (3rd ed). World Health Organization (WHO), Geneva. http://www.who.int/water_sanitation_health/dwq/GDW8rev1and2.pdf?ua=1. Accessed on 03 June 2016.
Woldehaimanot, B., & Behrmann, J. H. (1995). A study of metabasite and metagranite chemistry in the Adola region (South Ethiopia): implications for the evolution of the east African Orogen. Journal of African Earth Sciences, 21(3), 459–476. doi:10.1016/0899-5362(95)00098-E.
Worku, T., & Astin, T. R. (1991). The Karoo sediments (late Palaeozoic to early Jurassic) of the Ogaden Basin, Ethiopia. Sedimentary Geology, 76(1–2), 7–21. doi:10.1016/0037-0738(92)90136-F.
Worku, H., & Schandelmeier, H. (1996). Tectonic evolution of the Neoproterozoic Adola Belt of southern Ethiopia: evidence for a Wilson cycle process and implications for oblique plate collision. Precambrian Research, 77(3–4), 179–210. doi:10.1016/0301-9268(95)00054-2.
Wu, J., Li, P., Qian, H., Duan, Z., & Zhang, X. (2014). Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arabian Journal of Geosciences, 7, 3973–3982. doi:10.1007/s12517-013-1057-4.
Wu, J., Li, P., & Qian, H. (2015). Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environmental Earth Sciences, 73(12), 8575–8588. doi:10.1007/s12665-015-4018-2.
Yibas, B., Reimold, W. U., Armstrong, R., Koeberl, C., Anhaeusser, C. R., & Phillips, D. (2002). The tectonostratigraphy, granitoid geochronology and geological evolution of the Precambrian of southern Ethiopia. Journal of African Earth Sciences, 34(1–2), 57–84. doi:10.1016/S0899-5362(01)00099-9.
Yidana, S. M., Banoeng-Yakubo, B., & Sakyi, P. A. (2012). Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. Journal of Earth System Science, 121(2), 491–507. doi:10.1007/s12040-012-0163-0.
Zewdie, G., & Sima, J. (2011). Hydrogeological and hydrochemical maps of Negele NB 37–11: explanatory notes. Prague: Aquatest.
Acknowledgments
The authors thank the government of Ethiopia for the provision of fund, through the Ministry of Education, as support grant for this research. The cooperation of the National Meteorological Agency (NMA) in providing climatic data is also highly appreciated. The School of Earth Sciences of Addis Ababa University is also appreciated for arranging a vehicle for the field work and laboratory facilities for chemical and isotope analysis. The cooperation of zonal sector offices is highly acknowledged for their provision of secondary data and field information. Individuals including Uraga, Behailu and his family, Satana, Tarekegn, Gelegelo, and Adan are recognized for their support in data collection.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 10 kb)
Rights and permissions
About this article
Cite this article
Woldemariyam, F., Ayenew, T. Identification of hydrogeochemical processes in groundwater of Dawa River basin, southern Ethiopia. Environ Monit Assess 188, 481 (2016). https://doi.org/10.1007/s10661-016-5480-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5480-3