Abstract
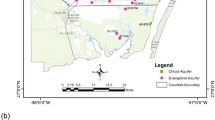
A hydrogeochemical study was conducted in the Dongsheng Coalfield, Ordos Basin, China, to identify the mechanisms responsible for the chemical compositions of the shallow groundwater and to document water quality with respect to agricultural and drinking supply standards, prior to mining. Tri-linear diagrams, principal component analysis, and correlation analysis were used to reveal the hydrogeochemical characteristics of the shallow groundwater, and the potential water–rock interactions. In general, the major cations and anions were present at low concentrations, but were relatively higher around Jiushenggong than elsewhere in the study area. Groundwater around Jiushenggong has a long residence time and is also subject to extensive evapotranspiration. The dominant hydrochemical facies are HCO3-Ca, HCO3-Na, and mixed HCO3-Ca·Na·Mg types. Increases in major ion concentrations along the flow path, including Na, Cl, and SO4, coincide with increases in total dissolved solids. The predominant mechanism controlling groundwater chemistry proved to be the dissolution of carbonates, gypsum, and halite. Cation exchange and mixing with local recharge water are also important factors. The shallow groundwater quality in the study area is suitable for agricultural and drinking purposes.
Zusammenfassung
Es wurde ein hydrochemische Studie im Kohlefeld Dongsheng (Ordos Becken, China) durchgeführt, um die Mechanismen aufzuklären, die für die chemische Zusammensetzung des flachen Grundwassers verantwortlich sind und um die Grundwasserqualität vor Beginn des Bergbaus zu dokumentieren, gemessen an Standards für die Wassernutzung in der Landwirtschaft und für die Trinkwasserversorgung. Dreiecksdiagramme, Hauptkomponentenanalyse und Korrelationsanalyse wurden benutzt, um die hydrochemische Charakteristik des flachen Grundwassers und die möglichen Wechselwirkungen Wasser-Gestein aufzuklären. Grundsätzlich waren die Konzentrationen der Hauptkationen und –anionen niedrig. Um Jiushenggong waren die Konzentrationen jedoch höher als im restlichen Untersuchungsgebiet. Das Grundwasser um Jiushenggong hat eine lange Aufenthaltszeit und ist außerdem durch hohe Evapotranspiration beeinflusst. Die dominierenden Grundwassertypen sind HCO3-Ca, HCO3-Na und gemischte HCO3-Ca*Na*Mg Typen. Die Zunahme der Konzentrationen der Hauptionen entlang des Fließweges, einschließlich Na, Cl und SO4, treten zusammen mit einem Anstieg der Gesamtkonzentration gelöster Stoffe auf. Die dominierenden Prozesse, die den Grundwasserchemismus bestimmen, sind die Auflösung von Karbonaten, Gips und Halit. Kationenaustausch und die Mischung mit der lokalen Grundwasserneubildung sind ebenfalls wichtige Faktoren. Die Qualität des flachen Grundwassers ist für landwirtschaftliche Nutzung und die Trinkwassergewinnung geeignet.
Resumen
Se realize un studio hidrogeoquímico en Dongsheng Coalfield, Ordos Basin, China, para identificar los mecanismos responsables de las composiciones químicas del agua subterránea superficial y documentar la calidad de agua con respecto a los estándares de agua potable y de agua para agricultura anteriores a la minería. Diagramas trilineales, análisis de componentes principales y análisis de correlación fueron usados para revelar las características hidrogeoquímicas del agua subterránea superficial y de las interacciones potenciales agua-roca. En general, los principales cationes y aniones estuvieron presentes en bajas concentraciones pero fueron relativamente mayores cerca de Jiushenggong. El agua subterránea cerca de Jiushenggong tiene un largo tiempo de residencia y está sujeto a evapotranspiración extensiva. Las fases hidroquímicas dominantes son HCO3-Ca, HCO3-Na y mezclas tipo HCO3-Ca*Na*Mg. Incrementos en las concentraciones de los iones mayoritarios a lo largo del flujo, incluyendo Na, Cl y SO4, coinciden con el total de sólidos disueltos. La disolución de carbonatos, yeso y halita provó ser el mecanismo que controla la química del agua subterránea. El intercambio de cationes y la mezcla con agua de recarga, son otros factores de importancia. La calidad del agua subterránea superficial en el área de estudio es adecuada para la agricultura y también para el consumo.
摘要
为了研究鄂尔多斯盆地东胜煤田煤矿开采前浅层地下水的水化学形成机制及其农业用水和饮用水水质状况,采用水文地球化学的理论,通过三线图、主成分分析和相关分析研究了浅层地下水的水文地球化学特征及其潜在的水岩作用。研究表明:总体上研究区地下水主要阴阳离子含量较低,但在九盛宫附近这些主要阴阳离子的含量相对较高。这主要是由于九盛宫附近浅层地下水径流缓慢,滞留时间较长且蒸发强烈。研究区浅层地下水主要以HCO3-Ca, HCO3-Na和HCO3-Ca·Na·Mg为主,主要离子浓度(包括Na+、Cl−和SO4 2−)沿流向不断增大,引起溶解性总固体也沿流向逐渐增大。碳酸盐、石膏和岩盐的溶解为控制地下水化学成分演化的主要机制。阳离子交换和局部补给水的混合也是影响地下水化学成分变化的重要因素。研究区浅层地下水水质适用于农业灌溉和饮用。






Similar content being viewed by others
References
Agartan E, Yazicigil H (2012) Assessment of water supply impacts for a mine site in western Turkey. Mine Water Environ 31:112–128. doi:10.1007/s10230-011-0167-z
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington
Bureau of Quality and Technical Supervision of China (1994) National standard of the People’s Republic of China: quality standard for groundwater, GB/T 14848-93 [in Chinese]
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering. Geochim Cosmochim Acta 8(1–2):22–48. doi:10.1016/0016-7037(55)90015-6
Dai SF, Ren DY, Chou CL, Li SS, Jiang YF (2006) Mineralogy and geochemistry of the no. 6 coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int J Coal Geol 66:253–270. doi:10.1016/j.coal.2005.08.003
Dassi L (2011) Investigation by multivariate analysis of groundwater composition in a multilayer aquifer system from North Africa: a multi-tracer approach. Appl Geochem 26:1386–1398. doi:10.1016/j.apgeochem.2011.05.012
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090. doi:10.1126/science.170.3962.1088
Han Y, Wang GC, Cravotta CA III, Hu WY, Bian YY, Zhang ZW, Liu YY (2012) Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China. Hydrol Process. doi:10.1002/hyp.9297
Hou GC, Zhang MS, Liu F, Wang YH, Liang YP, Tao ZP, Zhao ZH, Hu FS, Su XS, Lyu Y, Xie Y, Wang WK, Wang XY, Yang YY, Lu Q, Jiao YQ, Yang XC, Cui XD, Ma JL, Yin LH (2008) Groundwater investigation in ordos basin. Geologic Publishing House, Beijing [in Chinese]
Jolliffe IT (2002) Principal component analysis. Springer, New York City
Kaufman L (1990) Finding groups in data: an introduction to cluster analysis. Wiley, New York City
Lee RW (1981) Geochemistry of water in the Fort Union Formation of the Northern Powder River Basin, Southeastern Montana. USGS Water-Supply Paper 2076. US Government Printing Office, Washington
Li PY, Wu JH, Qian H (2010a) Groundwater quality assessment and the forming mechanism of the hydrochemistry in Dongsheng Coalfield of Inner Mongolia. J Water Resour Water Eng 21(1):38–41 [in Chinese]
Li PY, Qian H, Wu JH, Ding J (2010b) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3(3):282–291. doi:10.3882/j.issn.1674-2370.2010.03.004
Li PY, Qian H, Wu JH (2011a) Application of set pair analysis method based on entropy weight in groundwater quality assessment—a case study in Dongsheng City, Northwest China. E-J Chem 8(2):851–858. doi:10.1155/2011/879683
Li PY, Qian H, Wu JH (2011b) Hydrochemical formation mechanisms and quality assessment of groundwater with improved TOPSIS method in Pengyang County Northwest China. E-J Chem 8(3):1164–1173. doi:10.1155/2011/251918
Li PY, Wu JH, Qian H (2012) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci. doi:10.1007/s12665-012-2049-5
Lucas L, Jauzein M (2008) Use of principal component analysis to profile temporal and spatial variations of chlorinated solvent concentration in groundwater. Environ Pollut 151:205–212. doi:10.1016/j.envpol.2007.01.054
Marghade D, Malpe DB, Zade AB (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418. doi:10.1007/s10661-011-2126-3
Menció A, Mas-Pla J (2008) Assessment by multivariate analysis of groundwater–surface water interactions in urbanized Mediterranean streams. J Hydrol 352:355–366. doi:10.1016/j.jhydrol.2008.01.014
Ministry of Health of the PRC and Standardization Administration of the PRC (2006) Standards for drinking water quality (GB 5749–2006). China Standard Press, Beijing [in Chinese]
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321. doi:10.1016/j.chemosphere.2010.01.010
Niu CC, Wang Q, Wen XY, Guo Y, Zhagn PL, Zhu RY, He XH (2011) Application of principal component analysis to evaluation of black soil degradation in Jilin. Global Geol 14(1):54–58. doi:10.3969/j.issn.1673-9736.2011.01.07
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. USGS, Washington
PRC Ministry of Environmental Protection (2011) Technical guidelines for environment impact assessment-groundwater environment. China Environmental Science Press, Beijing
Qian H, Li PY (2011) Hydrochemical characteristics of groundwater in Yinchuan Plain and their control factors. Asian J Chem 23(7):2927–2938
RockWare Inc. (2004) A user’s guide to RockWare® Aq·QA®, version 1.1. RockWare Inc., Golden
Singh SK, Mahato MK, Neogi B, Singh KK (2010) Quality assessment of mine water in the Raniganj coalfield area, India. Mine Water Environ 29:248–262. doi:10.1007/s10230-010-0108-2
SPSS Inc. (2004) SPSS base 13.0 user’s guide. SPSS Inc., New York City
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41(5):552–562. doi:10.1007/s002540100431
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49(3):413–429. doi:10.1007/s00254-005-0089-9
Tóth J (1963) A theoretical analysis of groundwater flow in small drainage basin. J Geophys Res 68(16):4795–4812
United States Salinity Laboratory (USSL) (1954) Diagnosis and improvement of saline and alkali soils. US Dept of Agriculture (USDA), Agriculture Handbook 60, Washington, pp 69–81
Wilcox LV (1948) The quality of water for irrigation use. US Dept of Agriculture Tech Bull 1962, Washington
World Health Organization (WHO) (2011) Guidelines for drinking-water quality, 4th edn. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf. Accessed 24 Nov 2012
Xiao J, Jin ZD, Zhang F, Wang J (2012) Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. J Asian Earth Sci 52:21–30. doi:10.1016/j.jseaes.2012.02.006
Acknowledgments
The research was supported by the Doctor Postgraduate Technical Project of Chang’an University (CHD2011ZY025 and CHD2011ZY022), the Special Fund for Basic Scientific Research of Central Colleges (CHD2011ZY020 and CHD2011TD003), the National Natural Science Foundation of China (41172212, 51009009, and 41130753), and the special Funds for Scientific Research on Public Interest of the Ministry of Water Resources (201301084). Special thanks to Dr. Huang Wenfeng from Dalian University of Technology for his assistance in the field investigation. Sincere thanks to Dr. Charles A. Cravotta III, the associate editor of Mine Water and the Environment, for his editing of our manuscript, and Dr. Roger Lee, Dr. Wang Guangcai, and an anonymous reviewer for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

10230_2013_234_MOESM1_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM2_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM3_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM4_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM5_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM6_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)
Rights and permissions
About this article
Cite this article
Li, P., Qian, H., Wu, J. et al. Major Ion Chemistry of Shallow Groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32, 195–206 (2013). https://doi.org/10.1007/s10230-013-0234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-013-0234-8