Abstract
Vibrio species isolated from four different sampling stations in the west coast of Peninsular Malaysia were screened for their antimicrobial resistance and plasmid profiles. A total of 138 isolates belonging to 15 different species were identified. Vibrio campbellii, V. parahaemolyticus, V. harveyi, and V. tubiashii were found to predominance species at all stations. High incidence of erythromycin, ampicillin, and mecillinam resistance was observed among the Vibrio isolates. In contrast, resistance against aztreonam, cefepime, streptomycin, sulfamethoxazole, and sulfonamides was low. All the Vibrio isolates in this study were found to be susceptible to imipenem, norfloxacin, ofloxacin, chloramphenicol, trimethoprim/sulfamethoxazole, and oxytetracycline. Ninety-five percent of the Vibrio isolates were resistant to one or more different classes of antibiotic, and 20 different resistance antibiograms were identified. Thirty-two distinct plasmid profiles with molecular weight ranging from 2.2 to 24.8 kb were detected among the resistance isolates. This study showed that multidrug-resistant Vibrio spp. were common in the aquatic environments of west coast of Peninsular Malaysia.
Similar content being viewed by others
Introduction
Vibrios are ubiquitous in aquatic environments, depending on their salt requirement for optimum growth (Thompson et al. 2004). However, they tend to be more common in warmer waters, particularly in tropical waters (Wright et al. 1996). They are found free or in association with aquatic organisms (Reidl and Klose 2002; Thompson et al. 2005). The genus Vibrio comprises more than 63 species, of which about one third are potential human pathogens and have been implicated in water- and seafood-related outbreaks of gastrointestinal and wound infections in humans (Campos et al. 1996; Oliver and Kaper 1997; Thompson et al. 2004). The common pathogenic Vibrio species include Vibrio cholera, V. parahaemolyticus, V. fluvialis, V. mimicus, V.metschnikovii, V. hollisae (gastrointestinal tract infection), V. damsel, V. vulnificus, V. alginolyticus, V. lyticus, and V. furnissii (septicemias and wound infections) (Hlady et al. 1993; Campos et al. 1996; Oliver and Kaper 1997). Some Vibrio species are also known as zoonotic pathogens that cause diseases in marine animals (e.g., V. anguillarum, V. ordalii, V. salmonicida, V. splendidus, and V. harveyi) (Moriarty 1997; Vaseeharan and Ramasamy 2003; Jayasree et al. 2006).
V. cholera is the most important and well-studied strain. V. cholera is associated with both epidemic and pandemic diarrhea outbreaks in many parts of the world. Malaysia is located in the cholera endemic South East Asia zone and is usually associated with sporadic outbreaks (Mahalingam et al. 1994; Vadivelu et al. 2000; Radu et al. 2002). Data from the Infectious Diseases Division of the Ministry of Health Malaysia confirms the annual occurrence of cholera epidemics since 1980, and high prevalence was found between the years 1991 and 2011. To date, cholera still continues to pose a public health concern in Malaysia since there is an increase in cholera incidence from 0.34 per 100,000 populations in 2008 to 2.05 per 100,000 populations in 2011, with a mortality rate of 0.01 per 100,000 populations (Ministry of Health Malaysia 2011; WHO 2012).
In aquaculture industry, V. haryei, V. alginolyticus, V. mimicus, V. parahaemolyticus, and V. vulnificus are the common culprits in infectious diseases in marine aquaculture, e.g., Asian sea bass (Ransangan and Mustafa 2009), shrimps (Robertson et al. 1998), bivalves (Pass et al. 1987), and sea horse (Tendencia 2004). These vibrios have been reported to cause mortality and severe economic losses in aquaculture production countries (Sahul Hameed et al. 2003; Liu et al. 2004; Gopal et al. 2005). In Malaysia, vibriosis caused US$ 7.4 million losses in 1990 (Bondad-Reantaso et al. 2005). Antibiotics have been widely used to treat vibriosis in humans and aquaculture livestock. Based on the surveillance of antimicrobial resistance (Malla et al. 2014), the use of antibiotics in various clinical applications and aquaculture have resulted in the emergence of antibiotic-resistant bacteria and subsequently reduces the effectiveness of antibiotic to combat both human and animal infections. Another concern is the transfer of mobile genetic elements (e.g., plasmids, phophages, integrons, and transposons) and horizontal gene transfer (Serrano 2005) of antibiotic-resistant traits, which pose a potential global health risk. Vibrio species have been shown to have high similarity of genetic makeup of the environmental isolates and their pathogenic counterparts; therefore, Vibrio species have a high possibility to take up genes responsible for pathogenesis and also combine virulence genes (Chiang and Mekalanos 1999). Hence, Vibrio species may act as a reservoir for spreading of resistance genes in aquatic environments.
Most studies on the prevalence and antimicrobial susceptibility profiles of Vibrios to date have been focused on food and clinical samples. However, information on the occurrence of these bacteria in environmental sources is not well documented, especially in the tropical coastal waters. The present study evaluated the antibiotic susceptibility patterns and plasmid profiles of Vibrio spp. from four aquatic systems at the west coast of Peninsular Malaysia.
Materials and methods
Water sample collection and physical parameter measurements
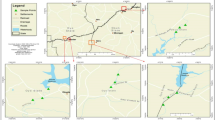
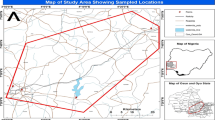
Water samples were collected from four different sampling sites along the west coast of Peninsular Malaysia, which are the hot spots for human activities. Port Dickson (PD) (2° 30′ N, 101° 50′ E) is a popular recreational coastal beach where beach resorts and food stalls are found along the coast. Port Klang (PK) (3° 0′ N, 101° 23′ E) is the major logistic hub in Malaysia and has been recognized as the largest multipurpose port in Southeast Asia. Port Klang is also located adjacent to the Klang Valley, which is a busy industrial and commercial center. Kuala Selangor (KS; 3° 21′ N, 101° 15′ E) is a fishing village located at the river mouth of Sungai Selangor. Sungai Muar (SM; 2° 3′ N, 101° 33′ E) is one of the major rivers in Malaysia and important water resources for drinking water; irrigation, agricultural, and industrial processes; and the recreational activity and mode of transportation for the community and traders. All samples were collected between May and December 2011, except water samples from KS which were collected in December 2010 and February 2011. The in situ physical parameters (temperature, pH, salinity, and dissolved oxygen) at each sampling site were measured using a Thermo Scientific Orion 5-Star Plus multiparameter meter, whereas Secchi depth was measured using a black and white Secchi disk. All collected water samples were kept in sterilized glass bottle and placed under cold conditions for no more than 3 h until processing in the laboratory.
Dissolved nutrient analysis and chlorophyll a measurement
For dissolved nutrient analysis, seawater samples were filtered through pre-combusted (500 °C for 3 h) GF/F filters (Whatman, UK) and stored at −20 °C until analysis. Dissolved inorganic nitrogen (nitrate [NO3], nitrite [NO2], and ammonium [NH4]), silicate (SiO2), and phosphate (PO4) concentrations were measured using a spectrophotometer (Parsons et al. 1984). Measurement of chlorophyll a was carried out according to Parsons et al. (1984). All measurements were carried out in triplicates.
Carlson’s trophic state index
The trophic state of the four sites was calculated according to Carlson (1977). Chlorophyll a concentration and Secchi depth were used to obtain the trophic state index (TSI) according to the equations
TSI values of less than 40 correspond to oligotrophic conditions, values between 40 and 50 indicate mesotrophic conditions, values of 50 to 70 indicate eutrophic environments, and index values greater than 70 are associated with hypertrophic conditions.
Isolation and identification of Vibrio species
The presumptive Vibrio strains were isolated from seawater samples by spread plating method (0.1 mL) and membrane filtration technique (1 mL) onto thiosulfate-citrate-bile salts-sucrose (TCBS) agar (Difco) supplemented with 2 % sodium chloride (NaCl). The inoculated plates were incubated at 30 °C overnight. The selected colonies were then purified and identified using biochemical and differential tests according to the Bergey’s Manual of Systematic Bacteriology (Murray et al. 1984) before being subjected to polymerase chain reaction (PCR) confirmation. The isolates which were confirmed were then separated into different operational taxonomic units (OTUs) based on their phenotypic characteristics by using Euclidean cluster analysis in PAST (version 2.17b). One representative isolate from each different OTUs was selected for 16S rDNA PCR using primers 341F (5′-CCTACGGGAGGCAGCAG-3′) and 907R (5′-CCGTCAATTCMTTTGAGTTT-3′), which produce an amplicon of approximately 586 bp. The procedure used in PCR was modified from that described by Winter et al. (2007). PCR conditions were as follow: initial denaturation at 95 °C for 1 min, followed by 30 cycles of 95 °C for 1 min, 62 °C for 1 min, 72 °C for 1 min, and a final extension step at 72 °C for 30 min. All sequences obtained were compared to GenBank entries using Basic Local Alignment Search Tool (BLAST) in order to obtain a preliminary affiliation.
Diversity index
The Vibrio species richness at four sites was determined using Shannon-Weaver index (H′), (Shannon and Weaver 1949). H′ was calculated with the following equation:
where Pi = ni/N (ni = number of Vibrio isolates of one species and N = total number of Vibrio tested).
Antibiotic susceptibility testing
The confirmed Vibrio species were tested for their susceptibilities to 14 antibiotics belonging to 10 different classes of antimicrobial agents. The tests were performed using E-test method and disk diffusion method as described by the Clinical and Laboratory Standards Institute (2005) on Mueller-Hinton agar (Difco) supplemented with 2 % NaCl. The minimum inhibitory concentrations (MICs) of antibiotics consisting of ampicillin (AM, 0.016–256 μg mL−1), aztreonam (AT, 0.016–256 μg mL−1), cefepime (PM, 0.016–256 μg mL−1), chloramphenicol (CL, 0.016–256 μg mL−1), erythromycin (EM, 0.016–256 μg mL−1), imipenem (IP, 0.002–32 μg mL−1), norfloxacin (NX, 0.016–256 μg mL−1), ofloxacin (OF, 0.002–32 μg mL−1), streptomycin (SM, 0.064–1024 μg mL−1), sulfamethoxazole (SX, 0.064–1024 μg mL−1), and trimethoprim/sulfamethoxazole 1/19 (TS, 0.002–32 μg mL−1) were determined by E-test strips (AB Biodisk, Sweden), and the MICs for mecillinam (Mel, 10 μg) and compound sulfonamides (S3, 300 μg) were determined by antibiotic disks (Oxoid, UK). The E-test strips and antibiotic disks were applied to the inoculated plates and incubated at 30 °C for 24 h under aerobic conditions. The MICs were determined according to the manufacturer’s instruction. For oxytetracycline (OTC), E-test produce unreliable results (Nonaka et al. 2000); therefore, the MIC was determined by 96-well microbroth dilution method (Clinical and Laboratory Standards Institute 2005). Isolates were grown overnight in tryptic soy broth supplemented with 2 % NaCl at 30 °C. Prior to inoculation, cell density was adjusted to an optical density (OD) of 0.5 at 625 nm. Twenty microliter of the bacterial suspension was inoculated into 96-well plates that contained 180-μL Mueller-Hinton broth (final cell density ~105 cfu mL−1; Difco, US) amended with 2 % NaCl and with twofold serial dilutions of OTC. The inoculated plates were incubated overnight at 30 °C. The MIC was determined as the minimum antimicrobial dilution which inhibits the visible growth of the bacteria being tested.
Multiple antibiotic resistance index
The average multiple antibiotic resistance (MAR) index of all Vibrio strains at one site was calculated by the formula
where “y” is the aggregate antibiotic resistance score of all Vibrio strains from one site, “n” is the number of Vibrio strains tested, and “x” is the number of antibiotics used in the study. A MAR index value of equal or greater than 0.2 indicates that antibiotic resistance at study area is rendered from high risk of contamination by antibiotics, whereas value smaller than 0.2 indicates that antibiotic resistance at study area is indigenous (Krumperman 1983).
Plasmid DNA isolation
Plasmid DNA was isolated from Vibrio species that were resistant to two or more antibiotic classes. Plasmid DNA isolation was performed according to O’Sullivan and Klaenhammer (1993). The isolated plasmids were checked via 0.8 % agarose gel electrophoresis. Alpha Imager 2200 was used to enumerate the sizes of plasmids by comparing with supercoiled DNA ladder (New England BioLabs). Escherichia coli pUC18 and E. coli pcDNA were used as positive controls.
Statistical analyses
Multivariate analysis of variance (MANOVA) and analysis of similarity were carried out according to Zar (1999). All data were reported as mean ± standard deviation (SD).
Results and discussion
The physico-chemical parameters obtained at our four sampling stations are shown in Table 1. The average surface water temperature of all sampling sites was 29.6 °C which is typically tropical waters (Bong and Lee 2008). Salinity varied over a wide range from 3.7 ± 3.6 to 27.5 ± 1.4 ppt, whereas pH ranged from 6.5 ± 0.8 to 7.9 ± 0.1. The chlorophyll a varied within a narrow range, except at Kuala Selangor where the chlorophyll a concentration was fivefold higher than other sampling stations. Both dissolved oxygen level and Secchi depth transparency were higher in coastal water (Port Dickson) compared to estuaries (Port Klang and Kuala Selangor) and river (Sungai Muar). All the dissolved inorganic nutrients were higher in estuaries and river, except for phosphate. From the physico-chemical properties, the four study sites were different (MANOVA, F = 2.32 × 1046, p < 0.01). The concentration of dissolved inorganic nutrients was within the range previously reported (Bong and Lee 2008; Lee and Bong 2008; DID 2009). The high levels of eutrophication were found in both estuarine (Kuala Selangor, Carlson’s TSI = 68) and river (Sungai Muar, Carlson’s TSI = 56). The major causes of eutrophication in these waters are land clearing (deforestation) and rapid development of agriculture, residential, commercial, industrial, and infrastructure in these areas (Ishak et al. 2003; Lee et al. 2006; DOE 2010).
Abundance and diversity of Vibrio species
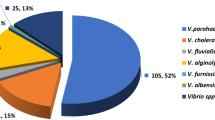
The Vibrio abundance varied from <1 ± 0 Est to 245 ± 58 cfu mL−1, and the highest abundance was detected in Kuala Selangor (Fig. 1). One-hundred and thirty-eight Vibrio isolates were obtained from this study, of which 48 isolates were from Port Dickson, 33 isolates from Port Klang, 34 isolates from Kuala Selangor, and 23 isolates from Sungai Muar. For the total number of isolates, 15 different Vibrio species were identified, with V. campbellii (n = 32, 23 %), V. parahaemolyticus (n = 32, 23 %), and V. harveyi (n = 17, 12.2 %) as the predominant species, followed by V. tubiashii (n = 12, 8.6 %), V. vulnificus (n = 10, 7.2 %), V. ponticus (n = 9, 6.5 %), and V. neptunius (n = 6, 4.3 %). The other species present were rare (less than 5 %), V. fortis, V. brasiliensis, V. cholerae, V. mediterranei, V. alginolyticus, V. azureus, V. sinaloensis, and V. xuii. In this study, the Shannon diversity index (H′) ranged from 0.9 to 2.2. The highest H′ was observed at Port Dickson (H′ = 2.2) followed by Port Klang (H′ = 1.5), Kuala Selangor (H′ = 1.6), and Sungai Muar (H′ = 0.9). Among the vibrios, V. campbellii, V. parahaemolyticus, V. harveyi, and V. tubiashii were found at all sampling sites (Fig. 2).
The abundance of Vibrio species measured in this study was within the range reported in other studies (Eiler et al. 2006; Wetz et al. 2008; Vijayan and Lee 2014). Our results showed that the average abundance and diversity of Vibrio were higher in estuarine and coastal waters compared to river water. This may be caused by the different characteristics of the water, particularly salinity (Eiler et al. 2006) and nutrient concentration (Motes et al. 1998; Eiler et al. 2006). Previous culture-dependent studies in temperate and tropical waters have shown to have a significant correlation between salinity, nutrient concentration, and the Vibrio spp. abundance (Thompson et al. 2004; McDougald and Kjelleberg 2006; Soto and Gutierrez 2009; Vijayan and Lee 2014). However, temperature is less important factor for tropical waters because temperature fluctuates less throughout the year compared to temperate waters (Turner et al. 2009; Asplund et al. 2011).
In this study, we compared the vibrio community structure via analysis of similarity and found that they were not significantly different among the four stations (p > 0.05). We found that V. campbellii, V. parahaemolyticus, and V. harveyi were the predominant species in all the sampling sites. These Vibrio species are commonly detected in tropical marine regions and are among the most important bacterial pathogens of many commercially farmed marine invertebrate and vertebrate species in many Asian countries (Vaseeharan and Ramasamy 2003; Gopal et al. 2005; Tanil et al. 2005). The prevalence of these pathogenic species in coastal, estuary, and river waters of west coast of Peninsular Malaysia may pose a significant health hazard to local individuals who have direct contact with water through recreational activities or via seafood consumption.
Antibiotic susceptibility testing
Of the 138 Vibrio isolates, only 84 isolates could be revived from −80 °C glycerol stocks (Port Dickson 28, Port Klang 22, Kuala Selangor 23, and Sungai Muar 11) and tested for their antibiotic susceptibility against 14 antibiotics (Fig. 3). The highest frequencies of resistance were observed against erythromycin (81.8–95.7 %), ampicillin (42–82 %), and mecillinam (42–55 %) at all sampling sites except for Sungai Muar where ampicillin (27 %) and mecillinam (9 %) resistance were low. The rates of resistance against aztreonam and cefepime were low (8.7–32 and 0–9 %, respectively) at all sampling sites. In contrast, low rate of streptomycin resistant was only found in isolates from both Port Dickson and Kuala Selangor, whereas low rate of sulfamethoxazole resistant was found in Kuala Selangor and Sungai Muar. At Sungai Muar, low resistance rate to sulfonamides was also observed. All the Vibrio isolates were found to be susceptible to imipenem, norfloxacin, ofloxacin, chloramphenicol, trimethoprim/sulfamethoxazole, and oxytetracycline.
Seventy-six (90.5 %) Vibrio isolates were found to be resistant to erythromycin with MIC range 1–12 μg mL−1. Moderate to high levels of ampicillin resistant were observed in 49 (58.3 %) isolates, 5 isolates with MIC range of 16–192 μg mL−1 and the remaining isolates showed higher MICs (≥256 μg mL−1). Aztreonam and cefepime resistance were found on a total of 19 (MIC range 16–≥256 μg mL−1) and 6 (MIC range 24–≥256 μg mL−1) isolates, respectively. The isolates from both Port Dickson and Sungai Muar were all found to have streptomycin resistance at a MIC of 32 μg mL−1. Moreover, high level of sulfamethoxazole resistance was observed in five isolates from both Kuala Selangor (MIC ≥1024 μg mL−1) and Sungai Muar (MIC range 512–≥1024 μg mL−1; Table 2).
The resistance of Gram-negative bacteria to erythromycin is expected due to their intrinsic resistance (Nikaido 1998), whereas ampicillin has been widely used since 1960 and ampicillin resistance is also commonly reported (e.g., Zanetti et al. 2001; Vaseeharan et al. 2005; Maluping et al. 2005; Akinbowale et al. 2006; Laganà et al. 2011). Mecillinam is a β-lactam antibiotic used in the treatment of urinary tract infections and has also been used for treatment of typhoid and paratyphoid fever (Clarke et al. 1976; Geddes and Clarke 1977). Mecillinam resistance is common among clinical isolates of E. coli, Shigella spp., and V. cholera O1 (Anderson 1977; Hossain et al. 1998). However, studies on mecillinam resistance in environmental isolates are less commonly reported. Our findings are consistent with Neela et al. (2007) who found a high percentage of mecillinam-resistant vibrios from seawater around fish cages in Japan. Mecillinam resistance in marine bacteria isolated from coastal waters of Peninsular Malaysia has also been reported (You et al. 2012). In the present study, the low resistance rates to ampicillin, sulfonamides, and mecillinam in Vibrio spp. isolated from Sungai Muar may due to the nutrient levels in the water. Studies with E. coli have shown that resistance to ampicillin, streptomycin, and sulfonamide were lost in phosphate-limited growth, whereas iron limitation has impact on mecillinam resistance (Godwin and Slater 1979; Vinella et al. 2005).
Aztreonam resistance among clinical and environmental Vibrio spp. is rarely reported (Jain et al. 2008; Pan et al. 2013). In this study, aztreonam resistance was 8.7–32.1 % and similar to other studies (24.2 % (Pan et al. 2013) and 12.5 % (Laganà et al. 2011)). In this study, six Vibrio isolates were found to be resistant to cefepime (7–9 %), which was higher than both Shaw et al. (2014) and Pan et al. (2013) who reported only 3 % of their Vibrio isolates as resistance to cefepime. The resistance extent of aztreonam and cefepime in this study is of concern as these antibiotics are considered to be some of the more effective defenses against severe infections caused by Vibrio. Moreover, cefepime is one of the newer fourth-generation cephalosporins. Thus, even a small percentage of resistance in environmental vibrios could raise significant concerns. Surveillance and monitoring of aztreonam and cefepime resistance vibrios in our aquatic environment are needed to reduce public health risks.
Sulfonamides (sulfa drugs) are most widely used class of antibiotics all over the world especially in developing Asian countries due to their inexpensiveness and wide spectrum antimicrobial activity (Managaki et al. 2007; Luo et al. 2011; Suzuki and Hoa 2012). These antibiotics are commonly used in agriculture, livestock operations, aquaculture, and human therapy. Resistance to sulfonamides has been reported in clinical, aquaculture, and aquatic environments from different geographical regions (Petersen et al. 2002; Kümmerer 2004; Hoa et al. 2008, 2011; You et al. 2012; Suzuki et al. 2013; Das et al. 2014). In this study, resistance against sulfonamides was only observed in Kuala Selangor (SX, 8.7 %) and Sungai Muar (SX and S3, 27.3 and 18.2 %, respectively), and their resistance rates were relatively lower compared to those studies observed in Philippines, Thailand, and Vietnam (Agersø and Petersen 2007; Hoa et al. 2011; Suzuki et al. 2013). The notable frequency of sulfonamide-resistant bacteria detected in both Kuala Selangor and Sungai Muar may have been due to inputs from domestic sewage, shrimp, and poultry farms in these areas that could promote the development of resistance. Although concentrations of antibiotics in the water samples were not measured in this study, several studies have reported the occurrence of selected veterinary antibiotics (macrolides, sulfonamides, fluroquinolones, and trimethoprim) in tropical waters (Managaki et al. 2007; Suzuki and Hoa 2012).
The antibiograms of all the 84 Vibrio isolates are shown in Table 3. Multiple antibiotic resistance was observed, and 20 different resistance antibiograms were identified among the isolates. Ninety-five percent of the Vibrio isolates were resistant to one or more different classes of antibiotic. Only 5 % were susceptible to all antibiotics tested, indicating widespread occurrence of MAR Vibrio spp. in coastal, estuary, and river waters in the west coast of Peninsular Malaysia. Most of the resistant isolates (48 %) were found to have resistance to two antibiotic classes, whereas 24 % were resistant against three antibiotic classes. One V. tubiashii strain isolated from Port Klang was found to be resistant to four different classes of antibiotic. None of the Vibrio isolates from water samples were fully resistant to all antibiotics used. Our results showed that Vibrio isolates from Kuala Selangor showed the highest frequency of MAR (83 %); these were followed by Port Klang (73 %), Port Dickson (68 %), and Sungai Muar (64 %). The MAR index of 0.2 was observed at all the sampling stations except Sungai Muar, which had a lower MAR index value of 0.1. In this study, there were no clear species-specific antibiotic resistance patterns. These results are in agreement with others studies showing multiresistance incidence in Vibrio spp. from aquatic environments and seafood samples (Neela et al. 2007; Matyar et al. 2008; Baker-Austin et al. 2009; You et al. 2012). The MAR indexes of Vibrio spp. from both coastal (Port Dickson) and estuary (Port Klang and Kuala Selangor) waters in this study were consistent to those Vibrio spp. isolated from seafood (Zulkifli et al. 2009; Noorlis et al. 2011) and other coastal waters (Tanil et al. 2005; Matyar et al. 2008), indicating that our coastal and estuarine waters are impacted by antibiotic pollution from human and animal sources.
Plasmid profiles
Forty-six strains (54.8 %) belonging to V. parahaemolyticus (18), V. campbellii (9), V. harveyi (7), V. ponticus (3), V. vulnificus (3), V. alginolyticus (2), V. azureus (2), V. cholerae (1), and V. tubiashii (1) were found to contain plasmids with molecular weight ranging from 2.2 to 24.8 kb, whereas 38 (45.2 %) isolates did not harbor any plasmids. Thirty-one isolates (36.9 %) had one plasmid, 8 isolates (9.5 %) had two, 4 isolates (4.8 %) had three, and 3 isolates (3.6 %) had four plasmids. Thirty-two distinct plasmid profiles were identified among the isolates (Table 3).
These findings were consistent with the study carried out by Zanetti et al. (2001) where 50 % of the Vibrio spp. isolated from coastal water harbor plasmids from 1.5 to 26 kb. The plasmids detected in this study were most probably not R plasmids as R plasmids are usually ≥30 kb (Guiney and Landa 1989). However, these small plasmids may still contribute to horizontal transfer via mobilization or conduction (Norman et al. 2009). Moreover, MDR strains detected in this study were without the presence of plasmids, suggesting that MDR-related genes in these Vibrio spp. can potentially be obtained through transposition, chromosomal-mediated conjugation among Vibrio spp., and other bacterial species (Son et al. 1998; Manjusha and Sarita 2011; Kitaoka et al. 2011). Besides, antibiotic resistance mechanisms including efflux pumps, mutation event, and sulfamethoxazole-trimethoprim (SXT) elements may also contribute to antibiotic resistance in vibrios (Levy and Marshall 2004; Nguyen et al. 2009; Kitaoka et al. 2011). More studies are needed to screen for other possible mobile genetic elements such as SXT element and integrons in order to understand the maintenance and dissemination of resistance determinants among Vibrio in tropical waters.
Conclusions
This study revealed that the antibiotic-resistant and multidrug-resistant Vibrio spp. were common in aquatic environments of west coast of Peninsular of Malaysia, suggesting a great risk to public health. R plasmid-mediated resistance was not observed, and further investigations are required to clarify the maintenance and mobility of these resistance determinants among Vibrio in tropical aquatic environments and how it might impact on human health and environment. Surveillance and monitoring of antibiotic resistance and pollution levels of antibiotics should be encouraged in order to reduce inappropriate use of antibiotics and the threat to public health.
References
Agersø, Y., & Petersen, A. (2007). The tetracycline resistance determinant Tet 39 and the sulphonamide resistance gene sulII are common among resistant Acinetobacter spp. isolated from integrated fish farms in Thailand. Journal of Antimicrobial Chemotherapy, 59, 23–27.
Akinbowale, O. L., Peng, H., & Barton, M. D. (2006). Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia. Journal of Applied Microbiology, 100, 1103–1113.
Anderson, J. D. (1977). Mecillinam resistance in clinical practice—a review. Journal of Antimicrobial Chemotherapy, 3(Suppl B), 89–96.
Asplund, M. E., Rehnstam-Holm, A. S., Atnur, V., Raghunath, P., Saravanan, V., Härnström, K., Collin, B., Karunasagar, I., & Godhe, A. (2011). Water column dynamics of Vibrio in relation to phytoplankton community composition and environmental conditions in a tropical coastal area. Environmental Microbiology, 13, 2738–2751. doi:10.1111/j.1462-2920.2011.02545.x.
Baker-Austin, C., McArthu, J. V., Lindell, A. H., Wright, M. S., Tuckfield, R. C., Gooch, J., Warner, L., Oliver, J., & Stepanauskas, R. (2009). Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microbial Ecology, 57, 151–159.
Bondad-Reantaso, M. G., Subasinghe, R. P., Arthur, J. R., Ogawa, K., Chinabut, S., Adlard, R., Tan, Z., & Shariff, M. (2005). Disease and health management in Asian aquaculture. Veterinary Parasitology, 32, 249–272.
Bong, C. W., & Lee, C. W. (2008). Nearshore and offshore comparison of marine water quality variables measured during SESMA 1. Malaysian Journal of Science, 27, 25–31.
Campos, E., Bolanos, H., Acuna, M. T., Diaz, G., Matamoros, M. C., Raventós, H., Sánchez, L. M., Sánchez, O., & Barquero, C. (1996). Vibrio mimicus diarrhea following ingestion of raw turtle eggs. Applied and Environmental Microbiology, 62, 1141–1144.
Carlson, R. E. (1977). A trophic state index for lakes. Limnology and Oceanography, 22, 361–369.
Chiang, S. L., & Mekalanos, J. J. (1999). Horizontal gene transfer in the emergence of virulent Vibrio cholerae. In E. Rosenberg (Ed.), Microbial ecology and infectious disease (pp. 156–169). Washington DC: ASM Press.
Clarke, P. D., Geddes, A. M., McGhie, D., & Wall, J. C. (1976). Mecillinam: a new antibiotic for enteric fever. British Medical Journal, 2(6026), 14–15.
Clinical and Laboratory Standards Institute. (2005). Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement M100-S15 (14th ed.). Wayne: CLSI.
Das, S. K., Rahman, A., Chisti, M. J., Ahmed, S., Malek, M. A., Salam, M. A., Bardhan, P. K., & Faruque, A. S. G. (2014). Changing patient population in Dhaka Hospital and Matlab Hospital of icddr, b. Tropical Medicine and International Health, 19(2), 240–243.
Department of Environment (DOE). (2010). Malaysia Environmental Quality report, 2010.
Department of Irrigation and Drainage (DID). (2009). Study on the river water quality trends and indexes in Peninsular Malaysia. Water Resources, 21.
Eiler, A., Johansson, M., & Bertilsson, S. (2006). Environmental influences on Vibrio populations in northern temperate and boreal coastal water (Baltic and Skagerrak Seas). Applied and Environmental Microbiology, 72, 6004–6011.
Geddes, A. M., & Clarke, P. D. (1977). The treatment of enteric fever with mecillinam. Journal of Antimicrobial Chemotherapy, 3(Suppl B), 101–102.
Godwin, D., & Slater, J. H. (1979). The influence of the growth environment on the stability of a drug resistance plasmid in Escherichia coli K 12. Journal of General Microbiology, 111, 201–210.
Gopal, S., Otta, S. K., Kumar, S., Karunasagar, I., Nishibuchi, M., & Karunasagar, I. (2005). The occurrence of Vibrio species in tropical shrimp culture environments: implications for food safety. International Journal of Food Microbiology, 102, 151–159.
Guiney, D. G., & Landa, E. (1989). Conjugative transfer of Inc plasmids. In C. M. Thomas (Ed.), Promiscuous plasmids of Gram-negative bacteria (pp. 27–56). London: Academic Press.
Hlady, W. G., Mullen, R. C., & Hopkin, R. S. (1993). Vibrio vulnificus from raw oysters. Leading cause of reported deaths from foodborne illness in Florida. The Journal of the Florida Medical Association, 80, 536–538.
Hoa, P. T. P., Nonaka, L., Viet, P. H., & Suzuki, S. (2008). Detection of the sul1, sul2, and sul3 genes in sulfonamide-resistant bacteria from wastewater and shrimp ponds of North Vietnam. Science of the Total Environment, 405, 377–384.
Hoa, P. H. P., Managaki, S., Nakada, N., Takada, H., Shimizu, A., Anh, D. H., Viet, P. H., & Suzuki, S. (2011). Antibiotic contamination and antibiotic-resistant bacteria in animal farms, city canal and aquaculture environments of North Vietnam. Science of the Total Environment, 409, 2894–2901.
Hossain, M. A., Rahman, M., Ahmed, Q. S., Malek, M. A., Sack, R. B., & Albert, M. J. (1998). Increasing frequency of mecillinam-resistant Shigella isolates in urban Dhaka and rural Matlab, Bangladesh: a 6-year observation. Journal of Antimicrobial Chemotherapy, 42, 99–102.
Ishak, B. H. O., Abdullah, F., & Soffie, W. M. (2003). National report of Malaysia on the formulation of a transboundary diagnostic analysis and preliminary framework of a strategic action programme for the Bay of Bengal. United Nations Environment Programme East Asian Regional Coordinating Unit.
Jain, M., Kumar, P., Goel, A. K., Kamboj, D. V., & Singh, L. (2008). Class 1 integrons and SXT elements conferring multidrug resistance in Vibrio cholerae O1 strains associated with a recent large cholera outbreak in Orissa, Eastern India. International Journal of Antimicrobial Agents, 32(5), 459–460.
Jayasree, L., Janakiram, P., & Madhavi, R. (2006). Characterization of Vibrio spp. associated with diseased shrimp from culture ponds of Andrha Pradesh (India). Journal of the World Aquaculture Society, 37, 523–532.
Kitaoka, M., Miyata, S. T., Unterweger, D., & Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae. Journal of Medical Microbiology, 60, 397–407.
Krumperman, P. H. (1983). Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Applied and Environmental Microbiology, 46(1), 165–170.
Kümmerer, K. (2004). Resistance in the environment. Journal of Antimicrobial Chemotherapy, 54, 311–320.
Laganà, P., Caruso, G., Minutoli, E., Zaccone, R., & Delia, S. (2011). Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela ssp. piscicida strains isolated from Italian aquaculture farms. The New Microbiologica, 34, 53–63.
Lee, C. W., & Bong, C. W. (2008). Bacterial abundance and production and their relation to primary production in tropical coastal waters of Peninsular Malaysia. Marine and Freshwater Research, 59, 10–21.
Lee, C. W., Bong, C. W., Ng, C. C., & Alias, S. A. (2006). Factors affecting variability of heterotrophic and phototrophic microorganisms at high water in a mangrove forest at Cape Rachado, Malaysia. Malaysia Journal of Science, 25, 55–66.
Levy, S. B., & Marshall, B. (2004). Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine, Suppl 10, S122–S129.
Liu, C. H., Cheng, W., Hsu, J. P., & Chen, J. C. (2004). Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Diseases of Aquatic Organisms, 61, 169–174.
Luo, Y., Xu, L., Rysz, M., Wang, Y., Zhang, H., & Alvarez, P. J. J. (2011). Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River basin, China. Environmental Science and Technology, 45, 1827–1833.
Mahalingam, S., Cheong, Y. M., Kan, S., Yassin, R. M., Vadivelu, J., & Pang, T. (1994). Molecular epidemiologic analysis of Vibrio cholera O1 isolates by pulsed-field gel electrophoresis. Journal of Clinical Microbiology, 32(12), 2975–2979.
Malla, S., Dumre, S. P., Shakya, G., Kansakar, P., Rai, B., Hossain, A., Nair, G. B., Albert, M. J., Sack, D., Baker, S., & Rahman, M. (2014). The challenges and successes of implementing a sustainable antimicrobial resistance surveillance programme in Nepal. BMC Public Health, 14, 269–275.
Maluping, R. P., Lavilla-Pitogo, C. R., DePaola, A., Janda, J. M., Krovacek, K., & Greko, C. (2005). Antimicrobial susceptibility of Aeromonas spp., Vibrio spp. and Plesiomonas shigelloides isolated in the Philippines and Thailand. International Journal of Antimicrobial Agents, 25, 348–350.
Managaki, S., Murata, A., Takada, H., Tuyen, B. C., & Chiem, N. H. (2007). Distribution of macrolides, sulfonamides and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environmental Science and Technology, 41, 8004–8010.
Manjusha, S., & Sarita, G. B. (2011). Plasmid associated antibiotic resistance in Vibrio isolated from coastal waters of Kerala. International Food Research Journal, 18, 1171–1181.
Matyar, F., Kaya, A., & Dinçer, S. (2008). Antibacterial agents and heavy metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Science of the Total Environment, 407(1), 279–285.
McDougald, D., & Kjelleberg, S. (2006). Adaptive responses to Vibrios. In F. L. Thompson, B. Austin, & J. Swings (Eds.), The biology of Vibrios (pp. 133–155). Washington, DC: American Society for Microbiology.
Ministry of Health Malaysia. (2011). Ministry of Health annual report (p. 69). Putrajaya: Ministry of Health Malaysia.
Moriarty, D. J. W. (1997). The role of microorganisms in aquaculture ponds. Aquaculture, 151, 333–349.
Motes, N. L., DePaola, A., Cook, D. W., Veazey, J. E., Hunsucker, J. C., Gartright, W. E., Blodgett, R. J., & Chirtel, S. J. (1998). Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Applied and Environmental Microbiology, 64, 1459–1465.
Murray, R. G. E., Brenner, D. J., Bryant, M. P., Holt, J. G., Kreig, N. R., Moulder, J. W., Pfennig, N., Sneath, P. H. A., & Staley, J. T. (1984). Bergey’s manual of systematic bacteriology. United States of America: Williams & Wilkins.
Neela, F. A., Nonaka, L., & Suzuki, S. (2007). The diversity of multi-drug resistance profiles in tetracycline-resistance Vibrio species isolated from coastal sediments and seawater. Journal of Microbiology, 45, 64–68.
Nguyen, D. T., Ngo, T. C., Tran, H. H., Nguyen, T. P., Nguyen, B. M., Morita, K., & Ehara, M. (2009). Two different mechanisms of ampicillin resistance operating in strains of Vibrio cholerae O1 independent of resistance genes. FEMS Microbiology Letters, 298(1), 37–43.
Nikaido, H. (1998). Multiple antibiotic resistance and efflux. Current Opinion in Microbiology, 1, 516–523.
Nonaka, L., Isshiki, T., & Suzuki, S. (2000). The occurrence of oxytetracycline resistant bacteria in the fish intestine and the seawater environment. Microbes and Environments, 15, 223–228.
Noorlis, A., Ghazali, F. M., Cheah, Y. K., Tuan Zainazor, T. C., Wong, W. C., Tunung, R., Nishibuchi, M., Nakaguchi, Y., Son, R., & Pui, C. F. (2011). Antibiotic resistance and biosafety of Vibrio cholera and Vibrio parahaemolyticus from freshwater fish at retail level. International Food Research Journal, 18(4), 59–66.
Norman, A., Hansen, L. H., & Sorensen, S. J. (2009). Conjugative plasmids: vessels of the communal gene pool. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364, 2275–2289.
O’Sullivan, D. J., & Klaenhammer, T. R. (1993). Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Applied and Environmental Microbiology, 59(8), 2730–2733.
Oliver, J. D., & Kaper, J. B. (1997). Vibrio species. In M. P. Doyle, L. R. Beuchat, & T. J. Montville (Eds.), Food microbiology (pp. 228–264). Washington, DC: American Society for Microbiology Press.
Pan, J., Zhang, Y., Jin, D., Ding, G., Luo, Y., Zhang, J., Mei, L., & Zhu, M. (2013). Molecular characterization and antibiotic susceptibility of Vibrio vulnificus in retail shrimps in Hangzhou, People’s Republic of China. Journal of Food Protection, 76(12), 2063–2068.
Parsons, T. R., Maita, Y., & Lalli, C. M. (1984). A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon Press.
Pass, D., Dybdahl, R., & Mannion, M. M. (1987). Investigations into the causes of mortality of the pearl oyster, Pinctada maxima (Jamson), in Western Australia. Aquaculture, 65, 149–169.
Petersen, A., Andersen, J. S., Kaewmak, T., Somsiri, T., & Dalsgaard, A. (2002). Impact of integrated fish farming on antimicrobial resistance in a pond environment. Applied and Environmental Microbiology, 68, 6036–6042.
Radu, S., Vincent, M., Apun, K., Abdul-Rahim, R., Benjamin, P. G., Yuherman, & Rusul, G. (2002). Molecular characterization of Vibrio cholerae O1 outbreak strains in Miri, Sarawak (Malaysia). Acta Tropica, 83, 169–176.
Ransangan, J., & Mustafa, S. (2009). Identification of Vibrio harveyi isolated from diseased Asian seabass Lates calcarifer by use of 16S ribosomal DNA sequencing. Journal of Aquatic Animal Health, 21(3), 150–155.
Reidl, J., & Klose, K. E. (2002). Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiology Reviews, 26, 125–139.
Robertson, P. A. W., Calderon, J., Carrera, L., Stark, J. R., Zherdmant, M., & Austin, B. (1998). Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Diseases of Aquatic Organisms, 32, 151–155.
Sahul Hameed, A. S., Rahaman, K. H., Alagan, A., & Yoganandhan, K. (2003). Antibiotic resistance in bacteria isolated from hatchery-reared larvae and post-larvae of Macrobrachium rosenbergii. Aquaculture, 217, 39–48.
Serrano, P. H. (2005). Responsible use of antibiotics in aquaculture (FAO fisheries technical paper. No. 469, p. 97). Rome: FAO.
Shannon, C. C., & Weaver, W. (1949). The mathematical theory of communication. Urbana: University of Illinois Press.
Shaw, K. S., Rosenberg Goldstein, R. E., He, X., Jacobs, J. M., Crump, B. C., & Sapkota, A. R. (2014). Antimicrobial susceptibility of Vibrio vulnificus and Vibrio parahaemolyticus recovered from recreational and commercial areas of Chesapeake Bay and Maryland coastal bays. PLoS ONE, 9(2), e89616.
Son, R., Nasreldine, E. H., Zaiton, H., Samuel, L., Rusul, G., & Nimita, F. (1998). Characterization of Vibrio vulnificus isolated from cockles (Anadara granosa): antimicrobial resistance, plasmid profile and random amplification of polymorphic DNA analysis. FEMS Microbiology Letters, 165, 139–143. doi:10.1111/j.1574-6968.1998.tb13138.x.
Soto, W., & Gutierrez, J. (2009). Salinity and temperature effects on physiological responses of Vibrio fischeri from diverse ecological niches. Microbial Ecology, 57(1), 140–150.
Suzuki, S., & Hoa, P. T. P. (2012). Distribution of quinolone, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Frontiers in Microbiology, 3, 67.
Suzuki, S., Ogo, M., Miller, T. W., Shimizu, A., Takada, H., & Siringan, M. A. (2013). Who possesses drug resistance genes in the aquatic environment? Sulfamethoxazole (SMX) resistance genes among the bacterial community in water environment of Metro-Manila, Philippines. Frontiers in Microbiology, 4, 102.
Tanil, G. B., Radu, S., Nishibuchi, M., Rahim, R. A., Napis, S., Maurice, L., & Gunsalam, J. W. (2005). Characterization of Vibrio parahaemolyticus isolated from coastal seawater in Peninsular Malaysia. The Southeast Asian Journal of Tropical Medicine and Public Health, 36(4), 940–945.
Tendencia, E. A. (2004). Short communication. The first report of Vibrio harveyi infection in the sea horse Hippocampus kuda Bleekers 1852 in the Philippines. Aquaculture Research, 35, 1292–1294.
Thompson, F. L., Iida, T., & Swings, J. (2004). Biodiversity of vibrios. Microbiology and Molecular Biology Reviews, 68, 403–431.
Thompson, F. L., Gevers, D., Thompson, C. C., Dawyndt, P., Naser, S., Hoste, B., Munn, C. B., & Swings, J. (2005). Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Applied and Environmental Microbiology, 71, 5107–5115.
Turner, J. W., Good, B., Cole, D., & Lip, E. K. (2009). Plankton composition and environmental factors contribute to Vibrio seasonality. ISME Journal, 3, 1082–1092.
Vadivelu, J., Iyer, L., Kshatriya, B. M., & Puthucheary, S. D. (2000). Molecular evidence of clonality amongst Vibrio cholerae O1 biotype El Tor during an outbreak in Malaysia. Epidemiology and Infection, 124, 25–30.
Vaseeharan, B., & Ramasamy, P. (2003). Abundance of potentially pathogenic microorganisms in Penaeus monodon larvae rearing systems in India. Microbiological Research, 158, 333–349.
Vaseeharan, B., Ramasamy, P., Murugan, T., & Chen, J. C. (2005). In vitro susceptibility of antibiotics against Vibrio spp. and Aeromonas spp. isolated from Penaeus monodon hatcheries and ponds. International Journal of Antimicrobial Agents, 26, 285–291.
Vijayan, M., & Lee, C. W. (2014). Seasonality and diversity of culturable vibrios in tropical coastal waters. Bulletin of Marine Science, 90(2), 599–610.
Vinella, D., Albrecht, C., Cashel, M., & D’Ari, R. (2005). Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Molecular Microbiology, 56, 958–970.
Wetz, J. J., Blacwood, A. D., Fries, J. S., Williams, Z. F., & Noble, R. T. (2008). Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River Estuary, North Carolina, during storm events. Aquatic Microbial Ecology, 53, 141–149.
Winter, C., Hein, T., Kavka, G., Mach, R. L., & Farnleitner, A. H. (2007). Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Applied and Environmental Microbiology, 73(2), 421–431.
World Health Organization (WHO). (2012). Weekly Epidemiological Report, 31–32, 289–304.
Wright, A. C., Hill, R. T., Johnson, J. A., Roghman, M. C., Colwell, R. R., & Morris, J. G. (1996). Distribution of Vibrio vulnificus in Chesapeake Bay. Applied and Environmental Microbiology, 62, 717–724.
You, K. G., Bong, C. W., & Lee, C. W. (2012). Antimicrobial resistance in bacteria isolated from tropical coastal waters of Peninsular Malaysia. Malaysian Journal of Science, 31(2), 111–120.
Zanetti, S., Spanu, T., Deriu, A., Romano, L., Sechi, L. A., & Fadda, G. (2001). In vitro susceptibility of Vibrio spp. isolated from the environment. International Journal of Antimicrobial Agents, 17, 407–409.
Zar, J. H. (1999). Biostatistical analysis (4th ed.). Upper Saddle River: Prentice Hall.
Zulkifli, Y., Alitheen, N. B., Raha, A. R., Yeap, S. K., Marlina, S. R., & Nishibuchi, M. (2009). Antibiotic resistance and plasmid profiling of Vibrio parahaemolyticus isolated from cockles in Padang, Indonesia. International Food Research Journal, 16, 53–58.
Acknowledgments
This work was financially supported by University of Malaya (UM RG192/12 SUS); Ministry of Science, Technology, and Innovation of Malaysia (MOSTI) (SF022-2013 and IOES 2014E); and Ministry of Higher Education (FP048/2013 A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, K.G., Bong, C.W. & Lee, C.W. Antibiotic resistance and plasmid profiling of Vibrio spp. in tropical waters of Peninsular Malaysia. Environ Monit Assess 188, 171 (2016). https://doi.org/10.1007/s10661-016-5163-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5163-0