Abstract
Phytophthora diversity was examined in eight forest and ornamental nurseries in the Czech Republic. A leaf baiting isolation technique and, in two nurseries, also Illumina DNA metabarcoding were used to reveal the diversity of Phytophthora in soil and irrigation water and compare the efficacy of both approaches. In total, baiting revealed the occurrence of 12 Phytophthora taxa in 59.4% of soil samples from seven (87.5%) nurseries. Additional baiting of compost was carried out in two nurseries and two Phytophthora species were recovered. Irrigation water was examined in three nurseries by baiting or by direct isolation from partially decomposed floating leaves collected from the water source, and two Phytophthora species were obtained. Illumina sequencing of soil and water samples was done in two and one nurseries, respectively. Phytophthora reads were identified as 45 Phytophthora taxa, 15 of them previously unknown taxa from Clades 6, 7, 8 and 9. Another 11 taxa belonged to known or undescribed species of the oomycete genera Globisporangium, Hyaloperonospora, Nothophytophthora, Peronospora and Plasmopara. Overall, with both techniques 50 Phytophthora taxa were detected with five taxa (P. taxon organica, P. plurivora, P. rosacearum, P. syringae and P. transitoria) being exclusively detected by baiting and 38 only by DNA metabarcoding. Particularly common records in DNA barcoding were P. cinnamomi and P. lateralis which were not isolated by baiting. Only seven species were detected by both techniques. It is recommended to use the combination of both techniques to determine true diversity of Phytophthora in managed or natural ecosystems and reveal the presence of rare or unknown Phytophthora taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora (Oomycota, Peronosporales) is a genus of mainly soilborne or aerial plant pathogens causing a range of diseases of woody plants like collar and root rots, aerial bleeding cankers on trunks, discolorations and small size of leaves or needles, and eventually dieback and mortality (Chen et al., 2022; Ho, 2018; Jung et al., 2013a, 2018a;). Phytophthora pathogens cause 66% of diseases affecting fine roots and over 90% of collar rot diseases of woody plants (Tsao, 1990). The worldwide count of significant Phytophthora diseases of forest trees has surged dramatically since the 1960s, rising from four to currently 41 (Brasier et al., 2022). Many species such as P. cactorum, P. cinnamomi, P. cryptogea, P. nicotianae, P. palmivora, P. ramorum and P. ×cambivora have very wide host ranges encompassing various taxa of woody plants (Erwin & Ribeiro, 1996; Oßwald et al., 2014). In nursery plants, Phytophthora pathogens cause root and collar rots leading to mortality and significant economic losses. Moreover, if the infection is suppressed by fungicides or fungistatic chemicals such as phosphites the infected plants often remain asymptomatic and after being planted in the wider environment cause significant losses in afforestations, reforestations, horticultural plantations, road-side plantings, parks, and private gardens (Frankel et al., 2020; Jung et al., 2016; Migliorini et al., 2015; Pérez-Sierra & Jung, 2013). Denman et al. (2009) described P. ramorum and P. kernoviae causing asymptomatic infections and sporulation on rhododendron and holm oak (Quercus ilex) leaves. Despite absence of necroses, both pathogens were regularly obtained from leaves using baiting tests. These findings are significant for phytosanitary services which base the regulation of these pathogens on visual inspection of plants (Denman et al., 2009).
Soilborne Phytophthora pathogens infect host plants primarily via the fine root system. In most cases, a successful infection begins with the chemotactical attraction of biflagellate zoospores to host roots. (Khew & Zentmyer, 1973). The zoospores adhere to the root surface where they encyst, secreting an adhesive substance that attaches them to the root epidermis (Hardham, 2001). A germ tube grows from the cyst, expands, and develops into hyphae that penetrate the root surface (Oh & Hansen, 2007). Initially, hyphae grow intercellularly, later intracellular hyphae are formed to obtain nutrition from living host cells.
Plants grown in greenhouses and nurseries play a significant role in spreading invasive pathogens and pests, which have a detrimental impact on ornamental plants, agricultural crops, and forest trees (Jung et al., 2016; Migliorini et al., 2015; Moralejo et al., 2009; Pérez-Sierra & Jung, 2013; Prospero et al., 2013). Phytophthora is often imported into nurseries with infected nursery stock, substrate, potting media or water used for irrigation. After being established in a nursery, inoculum is frequently disseminated through splashing irrigation water and horticultural techniques such as pruning, potting and moving plants. In some nurseries, the irrigation water is recycled and thus a constant circulation of the inoculum occurs (Bush et al., 2003, 2006; MacDonald et al., 1994; Pérez-Sierra & Jung, 2013; Themann et al., 2002).
Irrigation water is often taken from local surface water sources such as lakes, ponds, and rivers which have been demonstrated globally as important reservoirs of a broad spectrum of Phytophthora species and other oomycetes (Jung et al., 2017a, 2018b, 2019, 2020, 2022; Orlikowski et al., 2010, 2011, 2012; Shrestha et al., 2013). Research of plant diseases in commercial nurseries revealed that Phytophthora species can persist within the water recycling systems all year round (Bush et al., 2003; MacDonald et al., 1994; Themann et al., 2002). Thus, the use of effective techniques to disinfect irrigation water in commercial nurseries is crucial (Ufer et al., 2008).
People have engaged in trading and relocation of live plants for thousands of years (Maxwell et al., 2024). However, global trade is associated with risk of invasive alien species. The introduction of non-native pathogens into new environments is currently considered as the second most significant factor contributing to the decline in biodiversity, habitat destructions and rising economic costs (Brasier et al., 2022; Perrings et al., 2005; Pimentel et al., 2005).
Most – if not all – exotic invasive Phytophthora spp. causing epidemics of non-coevolved native vegetation were introduced via the international trade in living plants and subsequently spread with nursery stock (Brasier, 2008; Brasier et al., 2022; Chen et al., 2022; Jung et al., 2016, 2018a, 2021). For example, the devastating ‘Sudden oak death’ (P. ramorum) was imported to North America with nursery plants. In the United Kingdom P. ramorum has transitioned from rhododendrons to forest plantations of Larix kaempferi (Brasier et al., 2010, 2012). P. lateralis, first recorded in Seattle horticultural nurseries in the 1920s, has spread to the natural habitat of Chamaecyparis lawsoniana in Oregon and Northern California (Hansen, 2011; Hansen et al., 2000; Parke et al., 2014). The pathogen is now also responsible for decline among Chamaecyparis trees in Brittany (France) and Scotland. This epidemic was caused by the planting of trees from infected nurseries (Brasier et al., 2012; Green et al., 2020; Robin et al., 2011). Phytophthora ×alni in Europe, P. austrocedri in Chile and Argentina, and P. kernoviae in the United Kingdom are other Phytophthora spp. causing devastating forest epidemics with probable introduction by the nursery stock trade (Brasier et al., 2004, 2005; Greslebin et al., 2007; Hansen et al., 2012; Jung et al., 2016, 2018a, 2022; Jung & Blaschke, 2004; Parke et al., 2014).
A serious decline and dieback of European beech (Fagus sylvatica) stands caused by widespread fine root losses, root rot and aerial bleeding cankers has been observed across Europe in recent decades and 17 Phytophthora species have been obtained from affected European beech forests including several invasive species driving the disease, i.e. P. cactorum, P. plurivora and P. ×cambivora (Cacciola et al., 2005; Corcobado et al., 2020; Jung, 2009; Jung et al., 2005, 2013a, 2018a, 2019; Schmitz et al., 2009; Stępniewska & Dłuszyński, 2010; Telfer et al., 2015). In several countries, including Bavaria (Germany) and Austria, the initiation of the Phytophthora outbreak on beech was found associated with the alternation between extensive and long-lasting heavy rain and severe droughts during the vegetation period (Corcobado et al., 2020; Jung, 2009). Also, with the extensive decline of oak (Quercus spp.) forests across Europe an involvement of in total 22 Phytophthora taxa has been demonstrated with P. cinnamomi, P. plurivora, P. quercina and P. ×cambivora being most widespread and most aggressive to oak roots (Brasier et al., 1993; Jung et al., 1996, 2013a, 2018a, 2019, 2000; Seddaiu et al., 2020). In Southern Europe, the UK and the Czech Republic, P. cinnamomi and P. ×cambivora are causing, partly since the 1930s, the destructive ink disease of chestnut (Castanea sativa) trees (Day, 1938; Jung et al., 2018a; Vettraino et al., 2005). A study that took place in almost 2000 nursery stands and more than 2500 plantings across Europe showed almost omnipresent infestations of nurseries and young plantings of beech, chestnut, and oaks with a heterogenous mixture of Phytophthora species including those driving the declines of mature forest stands, i.e. P. cactorum, P. cinnamomi, P. plurivora, P. quercina and P. ×cambivora which were most common (Jung et al., 2016). In forest nurseries, P. cactorum is also causing damping-off of beech seedlings (Stepniewska, 2005; VÚLHM, 2007).
Surveys of Phytophthora infestation and diversity in both natural ecosystems and managed environments such as nurseries and plantations can be performed using baiting methods or metabarcoding approaches. Leaf baiting is a method that is widely used to detect Phytophthora pathogens. A soil sample is flooded with distilled water and young leaves (e.g. oak or beech) are placed on the water surface ca 2 cm above the soil. Zoospores released from sporangia produced in the the soil sample infect the floating leaves and cause necrosis. Phytophthora is then isolated from the necrotic tissue using a selective agar medium (Jung et al., 2000, 2013b, 2020). Baiting methods have long been used for isolation of Phytophthora from rhizosphere soil samples and occasionally also from root samples or bark cankers (Corcobado et al., 2020; Jung, 2009; Jung et al., 2013b; Pérez-Sierra et al., 2022). It helps to monitor Phytophthora diversity in natural or managed ecosystems (Balci & Halmschlager, 2003a; Balci & Halmschlager, 2003b; Jung et al., 2000, 2013b, 2017a, b, 2018b, 2019, 2020; Vettraino et al., 2002) or nurseries (Jung et al., 2016; Moralejo et al., 2009; Simamora et al., 2018). The advantage of baiting is the isolation of cultures, which are essential for taxonomic and genomic studies (Jung et al., 2016; La Spada et al., 2022). The success of baiting is impacted by many factors including the season and climatic conditions (Vannini et al., 2013); the interaction with other soil microorganisms, in particular the occurrence of faster-growing oomycetes like Pythium or Phytopythium spp. or antagonistic bacteria and fungi (Bose et al., 2018; Erwin & Ribeiro, 1996; Jung et al., 1996, 2000, 2017a, 2020; Pérez-Sierra et al., 2022); and quality of the baiting procedure and experience and skills of the research team. The compatibility of the Phytophthora species with the host bait leaves is also important (Sarker et al., 2023a, b) although Fagaceae leaves have proven effective in the isolation of far more than 100 known and many previously unknown Phytophthora taxa (Corcobado et al., 2020; Jung et al., 2000, 2016, 2017a, b, 2019; Seddaiu et al., 2020; Vettraino et al., 2002). Finally, the presence of dead or resting propagules cannot be detected by baiting (Catalá et al., 2015; La Spada et al., 2022). Phytophthora species exhibit differences in the timing and quantity of sporangia and zoospore production following baiting, and species that produce sporangia slowly may be challenging to identify because faster-sporulating species compete with them. In addition, Phytophthora species with slow growing mycelia, especially on selective agar media, can be overgrown by faster growing Pythium, Phytopythium or Phytophthora species (Jung et al., 2000, 2016, 2017a, 2018b, 2019, 2020; Pérez-Sierra et al., 2022; Sarker et al., 2021), hence getting pure cultures of slower growing Phytophthora species requires experience and special skills (Pérez-Sierra et al., 2022).
Metabarcoding studies are valuable for monitoring of invasive pathogens and diversity studies (Burgess et al., 2017; Catalá et al., 2015; Riddell et al., 2019). This method uses a highly specifc PCR assay followed by high throughput DNA sequencing in order to distinguish every species within a specified group (here Phytophthora and related oomycetes) present within any given environmental sample (Green et al., 2020; Mendoza et al., 2015). Studies conducted in various countries, including France, Ireland, Italy, Spain, Sweden and Australia (Burgess et al., 2017; Caballol et al., 2024; Catalá et al., 2015; La Spada et al., 2022; Redondo et al., 2018; Vannini et al., 2013) have proven the effectiveness of metabarcoding in the Phytophthora diversity studies in both soil and water. In all these studies, primers amplifying parts of the nuclear region spanning the internal transcribed spacer (ITS1–5.8S–ITS2) region of the ribosomal DNA were used since the ITS is one of the barcode regions of Phytophthora. However, metabarcoding detection of environmental DNA (eDNA) only confirms the existence of Phytophthora DNA within a specific environment. It does not provide information about whether viable individuals of each identified species are actually present (Burgess et al., 2017). Because smaller amount of soil is used for metabarcoding than for baiting, this may result in omission of some species which are present in the ecosystem but not in the soil sample tested (Sarker et al., 2023a). In contrast, metabarcoding of baiting leaves without symptoms of infection can be a useful additional technique for the monitoring of Phytophthora diversity (Sarker et al., 2023b).
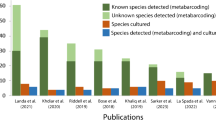
In studies that used both baiting and metabarcoding techniques, many more species were detected by metabarcoding compared with baiting (Catalá et al., 2015; Bose et al., 2018; Riddell et al., 2019; Landa et al., 2021; Sarker et al., 2023a; La Spada et al., 2022). However, to a lesser extent, metabarcoding approaches have also failed to detect Phytophthora species obtained by baiting (Sarker et al. 2023a).
In this study a survey of Phytophthora infestations of nurseries producing wide range of plant material (forest nurseries, nurseries producing large amenity trees and trees for landscape plantings and ornamental nurseries) in the Czech Republic was performed using and comparing baiting and metabarcoding methods.
Material and methods
Study areas and sampling of rhizosphere soil, compost, and irrigation water
A total of eight forest and ornamental nurseries were sampled across the Czech Republic (Table 1).
Forest nurseries produce only plants of forest trees for the state forestry and forestry enterprises in Czech Republic exclusively using seeds from the Czech Republic. No plants are imported from abroad. In contrast, ornamental nurseries produce a very wide spectrum of tree and shrub species, both native and non-native to the Czech Republic, for planting activities in private gardens and urban green areas. Plants are often imported from other European countries. Many ornamental nurseries also produce amenity trees which are grown for more years and to larger sizes. They are most often used for public parks and road-side plantings.
Sampling for baiting method
Soil samples were taken in all eight nurseries from trees and shrubs known to be susceptible to Phytophthora. Samples of compost, necrotic leaves floating in water ponds, and irrigation water were taken from selected sites (Table 2) because most nurseries did not have their own compost or did not have access to a surface water source.
At least three soil samples of each tree species per nursery were bulked and examined. Both symptomatic and asymptomatic plants were examined. In the case of bare root plants or containerised/potted plants older than five years, 3–5 soil samples were taken from opposing sides around the plant at a depth of 10 to 20 cm, depending on the age and size of the plant. Fine roots were also included in the soil sample, but coarser roots were avoided. The soil sub-samples for each individual plant were combined and mixed to create one bulked soil sample with a volume of approximately 0.5–1.0 L. In case of containerised plants younger than five years, 3–5 plants were removed from their pots and their rhizosphere soil pooled and mixed in a 1 L plastic bag.
If the nursery was using compost for preparing their soil substrate, samples were also taken from 3 to 5 different sites of a compost pile and pooled to a bulked compost sample of ca 0.5–1.0 L volume.
Irrigation water was sampled in nursery no. 3. Since this nursery was using both river and a reservoir as water sources and sand water filters, water samples of 10 L per sample were taken directly from the river and the reservoir and also after the filtration treatment.
Furthermore, in nurseries nos. 1, 3 and 8 fallen necrotic leaves floating on irrigation water reservoirs were sampled for detection of Phytophthora presence (natural baiting) according to Jung et al. (2017a, b, c). The collected leaves were immediately processed in the laboratory as described below for baiting leaves without the need to carry out baiting with fresh leaves.
Sampling for direct DNA extraction and metabarcoding
For detailed analyses by DNA metabarcoding two nurseries were selected with expected high (nursery no. 3) and low (nursery no. 8) Phytophthora diversity, respectively, according to different management practices. Nursery no. 8 is a forest nursery propagating nursery stock of local origin and not importing plants from abroad. In contrast, nursery no. 3 is focused on the propagation and sale of ornamental tree species, often not native to the Czech Republic, which are regularly imported from various European countries. Moreover, nursery no. 3 occupies a larger area than nursery no. 8.
NGS was only performed with water samples from nursery no. 3. Since this nursery has a water filter system, samples were taken before (water from river) and after the filtration. One sample was taken before filtration (from the river) and four samples from different parts of irrigation system after the filtration. The four samples were not mixed.
Isolation by baiting
Baiting commenced on either the day of sampling or within 24 hours of sampling and was therefore carried out with moist soil samples. Each soil or compost sample was placed in a plastic box 20 × 15 × 30 cm and flooded with distilled water to a depth of 3–4 cm above the soil surface. Litter and debris floating to the surface of the water was removed using a paper towel. After ca 2 hours of sedimentation, young soft leaves (without cuticle) of F. sylvatica and Q. suber were floated on the water and the baiting trays were incubated in a room with daylight at 18–20 °C (Jung, 2009; Pérez-Sierra et al., 2022).
Leaves becoming blackish or showing necrotic spots were briefly dried on a paper towel and small 2–3 mm pieces cut from the necroses were plated onto selective PARPNH – agar (Jung et al., 2017a) and incubated at 20 °C in the dark.
Baiting of irrigation water was conducted similarly. The irrigation water from the nursery was poured into a plastic box 20 × 15 × 30 cm and the water surface was covered with young Fagus and Quercus leaves as in the soil baiting. Leaves developing necrotic lesions were processed as described before.
Naturally fallen necrotic leaves floating in irrigation water reservoirs were used as natural baits and taken to the laboratory, where they were briefly dried and processed as described before (Jung et al., 2020).
PARPNH – Petri dishes with plated leaf pieces were examined daily under stereomicroscope at ×20 magnification for mycelial growth. When Phytophthora hyphae were observed an agar plug with a few hyphae from the growing margin of the colony was cut out and transferred onto vegetable juice agar (V8A) or carrot juice agar (CA) (Jung et al., 1996, 2017a; Pérez-Sierra et al., 2022).
All oomycete isolates were deposited in the culture collection of the Phytophthora Research Centre at Mendel University in Brno.
DNA extraction from mycelia and identification of Phytophthora isolates
DNA from mycelia of clean isolates obtained from baiting (or by cultivating naturally fallen necrotic leaves on selective agar) was extracted according to a modified protocol of Lamour and Finley (2006) optimised by Jung et al. (2023). DNA was then purified using the Monarch Genomic DNA Purification Kit (New England Biolabs, Ipswich, USA) and treated with RNase A following the manufacturer’s protocol for tissue samples. DNA was eluted with 100 μL of pre-warmed elution buffer and preserved at −80 °C for long term storage.
Identification of Phytophthora cultures by sequencing of the nuclear region spanning the internal transcribed spacer (ITS1–5.8S–ITS2) region of the ribosomal DNA and the mitochondrial Cox1 gene was performed according to Jung et al. (2020). The ITS region was amplified using primer-pairs ITS1/ITS4 or ITS6/ITS4 (Cooke et al., 2000; White et al., 1990). The Cox1 gene was amplified with both primer-pairs COXF4N/COXR4N and FM84/FM83 (Kroon et al., 2004; Martin & Tooley, 2003). The PCR reaction mixture and the amplification conditions for ITS and Cox1 were according to Cooke et al. (2000), Martin and Tooley (2003) and Kroon et al. (2004). All amplicons were purified and sequenced in both directions by Eurofins Genomics GmbH (Cologne and Ebersberg, Germany) using the amplification primers.
For species identification, consensus sequences were blasted against GenBank (http://www.ncbi.nlm.nih.gov/BLAST/) and a local database containing sequences of ex-type isolates and reference isolates from published studies. Isolates were assigned to a species when their sequences were at least 99% identical to a reference isolate.
DNA extraction from rhizosphere soil and water samples
DNA from soil was extracted using DNeasy PowerLyzer PowerSoil Kit (QIAGEN). Three replicates per soil or water sample were processed. Each sample was divided into three homogenisation tubes included in the kit and the kit protocol was followed without modifications.
After the extraction, the DNA was purified using Monarch Genomic DNA Purification Kit (New England Biolabs, Ipswich, USA).
This DNA was subsequently used for nested PCR and DNA metabarcoding.
PCR
For DNA metabarcoding a nested PCR assay to amplify the ITS1 region was conducted in accordance with the protocol of Riddell et al. (2019) also used in La Spada et al. (2022) using the primer pairs 18Ph2F (5′-GGATAGACTGTTGCAATTTTCAGT-3′) and 5.8S-1R (5′-GCARRGACTTTCGTCCCYRC-3′) (Scibetta et al., 2012) in the first round and ITS-6 (5′-GAAGGTGAAGTCGTAACAAGG-3′) (Cooke et al., 2000) and 5.8S-1R in the second round. The second-round primers were modified with overhang adapters for compatibility to the Illumina index and sequencing adapters. These were: forward overhang; 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-3′ (ITS-6) and reverse overhang; 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3′ (5.8S-1R) (Illumina Inc., 2013). In each reaction, hi-fidelity KAPA HiFi DNA Polymerase (Roche SA, Basel, Switzerland) was used. Control reactions were included that comprised a mix of four synthetic DNA oligonucleotides with an equivalent length to the Phytophthora ITS1 region and amplification primers (ITS-6 and 5.8S-1R) included at each end (GenBank accession numbers PP407413 to PP407416). The amplicons obtained were then run on 1.5% agarose gels and all oomycete-positive PCR products were selected for downstream processing.
Illumina sequencing library preparation and sequencing
Library preparation was done according to La Spada et al. (2022). In brief, PCR products were prepared for Illumina metabarcode sequencing following the instructions reported in the protocol 16S Metagenomic Sequencing Library Preparation (Illumina Inc., 2013). The obtained amplicons were subjected to a PCR clean-up using the Agencourt® Ampure® XP beads kit (Agencourt Bioscience, Beverly, MA, USA) and then moved to Index PCR using the Nextera XT Index Kit (Epicentre, Madison, WI, USA) to attach dual indices and Illumina sequencing adapters to each amplicon. This step was to allow the association of each unique index and amplicon to a single sample after the sequencing run. A second PCR clean-up was then run, as above, and the final PCR products from each sample were visualized on a 1.5% agarose gel. All the products were then quantified by fluorometry with the Quant-iT™ PicoGreen™ dsDNA Assay Kit (Invitrogen™, Waltham, MA, USA) and pooled to a single library that was adjusted to a concentration of 4 nM. The libraries were sequenced at the James Hutton Institute (Dundee, United Kingdom) using the MiSeq version 2 chemistry with 500 cycles (reagent kit MS-102-2003, Illumina, San Diego, CA, USA). The obtained FASTQ files containing barcode reads for each sample were then exported for bioinformatic analysis.
Analyses of Illumina data
The Illumina paired end reads were processed using version 0.12.1 of the Tree Health and Plant Biosecurity Initiative Phytophthora ITS1 Classifier Tool THAPBI PICT open-source software pipeline for metabarcoding analysis (Cock et al., 2023). The core of the analysis involves data reduction to unique marker sequences termed amplicon sequence variants (ASVs) which are then processed against dynamic thresholds based on control samples on the Illumina flow cell. Then ASVs are classified against a curated reference database of THAPBI PICT Phytophthora ITS1 and a local Phytophthora Research Centre database containing sequences of ex-type or key isolates from published studies (Aghighi et al., 2012; Cooke et al., 2000; Jung et al., 2019, 2020; Riolo et al., 2020; Safaiefarahani et al., 2015; Santilli et al., 2020; Scanu et al., 2014) using BioEdit. ASVs were assigned to a species when their sequences were at least 99% identical to a reference sequence. Any sequences not assigned to a species from the reference databases were classified at the genus or clade level. The phylogenetic relationship of aligned dataset of detected sequences was analysed by means of the maximum likelihood algorithm carried out using Phylogeny.fr (http://phylogeny.lirmm.fr/phylo_cgi/index.cgi) the “A la Carte” Mode (PhyML, GBlock disabled, GTR evolutionary model, bootstrapping procedure with 100 replicates).
Results
Species detected by baiting isolation and ITS sequencing from soil
Using a baiting technique Phytophthora spp. were isolated from 38 of the 64 rhizosphere soil samples (59.4%) tested from seven of the eight nurseries sampled. In nursery no. 6 no Phytophthora isolates were recovered. Overall, 94 Phytophthora isolates were obtained which, acording to ITS sequence analysis, belonged to 12 Phytophthora taxa (Table 2). P. plurivora was most commonly isolated (26 isolates, 7 host species, 6 nurseries) followed by P. cryptogea (26 isolates, 6 host species, 3 nurseries). Phytophthora plurivora was isolated regularly from Fagus and Quercus, but also from Berberis, Buxus, Viburnum, Alnus and Magnolia, whereas P. cryptogea was found on Fagus, Quercus, Viburnum, Ulmus and the conifers Picea and Pinus. Interestingly, these two species never co-occurred in the same soil sample.
A previously unknown Phytophthora species from phylogenetic Clade 13 was obtained during this survey from seedlings of Quercus robur in nursery no. 3 and described in 2022 as Phytophthora transitoria (Abad et al., 2023; Chen et al., 2022).
Compost
Compost was tested in nurseries no. 4 and no. 7 and P. cryptogea and P. gonapodyides were isolated from both nurseries (Table 3).
Irrigation water
Irrigation water was tested in three nurseries by baiting or by direct isolation from necrotic leaves collected from the water source. Only P. lacustris and P. gonapodyides were isolated from 3 and 2 nurseries, respectively (Table 3).
Species detected by Illumina sequencing from eDNA
Illumina metabarcoding was completed on samples from nurseries nos. 3 and 8 with a total of 14 eDNA samples (five from water, nine from soil) which produced positive nested PCR amplicons suitable for metabarcoding. A plate threshold of 422 reads was established on the basis of control samples, meaning only sample ASVs with read numbers above 422 were considered in the following results.
Overall, ITS1 barcodes corresponding to 45 Phytophthora taxa were detected from soil and water samples, 15 of them undescribed Phytophthora taxa from Clades 6, 7, 8, 9. Another 11 taxa belonged to both known and unknown species of Globisporangium, Hyaloperonospora, Nothophytophthora, Peronospora and Plasmopara.
Soil samples
Abundance, i.e. the number of samples in which a particular species was detected and read abundance, i.e. the number of metabarcode reads of a specific species, are shown in Figs. 1 and 2, respectively.
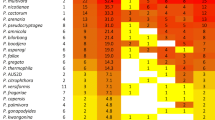
Abundance of Phytophthora taxa identified in soil samples from two nurseries (blue = ornamental, red = forest) by DNA metabarcoding. ALE/ALP/CAC = P. aleatoria/P. alpina/P. cactorum; AUS = P. t. AUS9C; ×CAM = P. ×cambivora; CAP = P. aff. capsici; CAP/GLO = P. capsici/P. glovera; CAS/QUE/VERS = P. castanetorum/P. quercina/P. versiformis; CIN = P. cinnamomi; CIT = P. citrophthora; CON = P. condilina; CRA/MEG = P. crassamura/P. megasperma; HYD/PAR = P. hydropathica/P. parsiana; CHL/×STA = P. chlamydospora/P. ×stagnum; INU = P. inundata; KWO = P. kwongonina; LAT = P. lateralis; MIS = P. mississippiae; MEG = P. megasperma; ×MUL = P. ×multiformis; ORN = P. ornamentata; PSE = P. pseudosyringae; PSY = P. psychrophila; P.t. 1–13 = Phytophthora taxon 1–13 (novel taxons); SAN = P. sansomeana; ×STA = P. ×stagnum; SUL = P. sulawesiensis; UNI = P. uniformis; VIR = P. virginiana
Read abundance of Phytophthora taxa identified in soil samples from ornamental nursery no. 3 by DNA metabarcoding. ALE/ALP/CAC = P. aleatoria/P. alpina/P. cactorum; AUS = P. t. AUS9C; ×CAM = P. ×cambivora; CAP = P. aff. capsici; CAP/GLO = P. capsici/P. glovera; CAS/QUE/VERS = P. castanetorum/P. quercina/P. versiformis; CIN = P. cinnamomi; CIT = P. citrophthora; CON = P. condilina; CRA/MEG = P. crassamura/P. megasperma; HYD/PAR = P. hydropathica/P. parsiana; CHL/×STA = P. chlamydospora/P. ×stagnum; INU = P. inundata; KWO = P. kwongonina; LAT = P. lateralis; MIS = P. mississippiae; MEG = P. megasperma; ×MUL = P. ×multiformis; ORN = P. ornamentata; PSE = P. pseudosyringae; PSY = P. psychrophila; P.t. 1–13 = Phytophthora taxon 1–13 (novel taxon); SAN = P. sansomeana; ×STA = P. ×stagnum; SUL = P. sulawesiensis; UNI = P. uniformis; VIR = P. virginiana
Barcodes matching a total of 34 Phytophthora taxa were detected in soil samples from nurseries 3 and 8 (Figs. 1, 2 and 3; Table 4). In nursery no. 8, four bulked soil samples were tested. Two were from Pinus sylvestris plants of different ages and two from Fagus sylvatica plants of different ages. In this nursery very low diversity of Phytophthora species was found. Only ASVs associated with P. × cambivora on Pinus sylvestris and P. aleatoria/alpina/cactorum on Fagus sylvatica were found. In contrast, with 32 Phytophthora taxa, diversity was much higher in nursery no. 3. In this nursery, five bulked soil samples were taken from the species Berberis sp., Chamaecyparis pisifera, Buxus sp., and two samples from Euonymus sp. with different ages. With 19 Phytophthora species the highest diversity was found in soil from the rhizosphere of symptomatic Berberis plants. The Phytophthora species with the highest abundance in nursery no. 3 was P. cinnamomi (Figs. 1, 2 and 3), recorded from soil of all tested plants and all samples. Barcodes matching P. lateralis, a species previously not recorded from Czech Republic, was discovered with the second highest abundance in four soil samples from Chamaecyparis, Berberis, Buxus, and Euonymus. Two species, P. virginiana and P. uniformis were detected with the same abundance as P. lateralis in four out of five samples from this nursery. Another 20 known species or informally designated taxa of Phytophthora were found in nursery 3 including the globally distributed wide-host range pathogens P. citrophthora and P. megasperma and P. ×multiformis, together with P. uniformis one of the drivers of the devastating Alnus dieback along rivers in Europe. The findings of P. kwongonina, P. mississippiae, P. taxon sulawesiensis (exact match with GenBank accession no. EF590257), P. ×stagnum (all from Clade 6) and P. taxon AUS9C (exact match with GenBank accession no. KY110356.1) from Clade 9d are first records for Europe whereas P. condilina, P. ornamentata and P. sansomeana are for the first time reported from Czech Republic (Fig. 1). The relatively short ITS1 sequence produced by the metabarcoding primers could not discriminate taxa from closely related species complexes to the species level, i.e. P. aleatoria/P. alpina/P. cactorum, P. capsici/P. glovera, P. castanetorum/P. quercina/P. versiformis, P. chlamydospora/P. ×stagnum, P. crassamura/P. megasperma and P. hydropathica/P. parsiana (Fig. 1). Barcodes of an additional eight novel Phytophthora taxa from Clades 6, 7, 8 and 9 (Fig. 7), Nothophytophthora intricata and the downy mildew (DM) species Peronospora variabilis and Pe. cf. fagopyri were detected in nursery 3 (Table 4), whereas in nursery 8 three known Peronospora species, i.e. Pe. variabilis, Pe. conglomerata and Pe. mayorii, and three and two novel taxa of the DM genera Hyaloperonospora and Plasmopara, respectively, were detected (Table 4). The finding of N. intricata, originally described from the rhizosphere of a riparian Aesculus hippocastanum in Germany (Jung et al., 2017c), is the second record of this species and the first report of a Nothophytophthora species from a nursery environment. Table 4 describes differences in the species diversity in soil between nurseries no. 3 and no. 8.
Water samples
Abundance and read abundance are shown in Figs. 4, 5 and 6.
Abundance of Phytophthora taxa detected by DNA metabarcoding in water samples (green = river water before filtration, yellow = after filtration) from nursery no.3. ×CAM = P. ×cambivora; CAP/GLO = P. capsici/P. glovera; aff CAP = P. aff. capsici; CAS/QUE/VERS = P. castanetorum/P. quercina/P. versiformis; CIN = P. cinnamomi; CIT = P. citrophthora; CON = P. condilina; CRY = P. cryptogea; GIB/GRE = P. gibbosa/P. gregata; GON = P. gonapodyides; CHL/×STA = P. chlamydospora/P. ×stagnum; INU = P. inundata; KWO = P. kwongonina; LAT = P. lateralis; MEG = P. megasperma; ×MUL = P. ×multiformis; PSE = P. pseudosyringae; P.t. 1–15 = Phytophthora taxa 1–15 (novel taxa); SAN = P. sansomeana; ×STA = P. ×stagnum; UNI = P. uniformis; VIR = P. virginiana
Read abundance of Phytophthora taxa detected by DNA metabarcoding in filtered water samples from nursery no.3. ×CAM = P. ×cambivora; CAP/GLO = P. capsici/P. glovera; aff CAP = P. aff. capsici; CAS/QUE/VERS = P. castanetorum/P. quercina/P. versiformis; CIN = P. cinnamomi; CIT = P. citrophthora; CON = P. condilina; CRY = P. cryptogea; GIB/GRE = P. gibbosa/P. gregata; GON = P. gonapodyides; CHL/×STA = P. chlamydospora/P. ×stagnum; INU = P. inundata; KWO = P. kwongonina; LAT = P. lateralis; MEG = P. megasperma; ×MUL = P. ×multiformis; PSE = P. pseudosyringae; P.t. 1–15 = Phytophthora taxa 1–15 (novel taxa); SAN = P. sansomeana; ×STA = P. ×stagnum; UNI = P. uniformis; VIR = P. virginiana
Read abundance of Phytophthora taxa detected by DNA metabarcoding in unfiltered water samples from nursery no. 3. CIN = P. cinnamomi; CRY = P. cryptogea; CHL/×STA = P. chlamydospora/P. ×stagnum; INU = P. inundata; ×MUL = P. ×multiformis; P.t. 5–15 = Phytophthora taxon 5–15 (novel taxon); UNI = P. uniformis
With nine species the Phytophthora diversity was lower in the unfiltered natural river water (Fig. 4). Most common (highest number of reads) was P. cryptogea followed by P. ×multiformis, P. chlamydospora/P. ×stagnum and P. uniformis (Fig. 6). Interestingly, P. cinnamomi was also present in the river water.
In contrast, 21 known and 6 novel Phytophthora taxa were detected in the filtered water (Fig. 4). At least 15 Phytophthora taxa were present in each of the four tested water samples. The most common species was P. cinnamomi which appeared in all replicates (Fig. 4) with the highest total number of 38,435 reads (Fig. 5) followed by P. lateralis with 29,579 reads (Figs. 4 and 5). Overall, 14 Phytophthora taxa were present in all four replicates (Fig. 4). Phytophthora uniformis and P. castanetorum/P. quercina/P. versiformis had more than 15,000 reads whereas P. chlamydospora/P. ×stagnum and P. virginiana had more than 10,000 reads. The relatively short ITS1 sequence produced by the metagenomic primers did not allow the discrimination to species level for several taxa from closely related species complexes, i.e. P. chlamydospora/P. ×stagnum, P. capsici/P. glovera, P. castanetorum/P. quercina/P. versiformis, P. gibbosa/P. gregata and P. hydropathica/P. parsiana. Surprisingly, no other oomycete taxa were detected in any water sample (Fig. 7).
Comparison of results from baiting and DNA metabarcoding
Overall, 50 Phytophthora species were detected with both techniques in this study from soil and water samples of eight and three nurseries, respectively. Soil samples from nurseries no. 3 and 8 and water samples from nursery 3 were tested by both baiting and DNA metabarcoding permitting some comparisons between both techniques.
Soil samples
Nursery no. 8 showed low Phytophthora diversity with both methods but, it was noteworthy that no species overlap was detected and more species, i.e. P. plurivora, P. syringae and P. cactorum, were detected with baiting than with DNA metabarcoding, i.e. P. cactorum (or P. aleatoria or P. alpina) and P. ×cambivora. Nine known and unknown taxa from other oomycete genera Globisporangium, Hyaloperonospora, Nothophytophthora, Peronospora and Plasmopara were also detected in this nursery (Table 4) but only by metabarcoding and not by baiting.
In contrast, in nursery no. 3 the diversity detected by DNA metabarcoding was much higher compared to baiting. While baiting revealed only P. plurivora, P. transitoria and P. citrophthora, DNA metabarcoding detected 32 Phytophthora taxa including eight novel ones plus three known and unknown taxa from the oomycete genera Nothophytophthora and Peronospora.
Water samples
While baiting detected only P. gonapodyides and P. lacustris, both primarily aquatic species with almost ubiquitous distribution in European waterways, DNA metabarcoding unveiled a rich community of 22 known and eight previously unknown taxa of Phytophthora. Phytophthora gonapodyides was the only species detected with both techniques. Surprisingly, neither P. lacustris nor any other oomycete genus were detected by metabarcoding of water samples.
Discussion
Previous studies have demonstrated a high diversity of Phytophthora populations in both natural and managed ecosystems across Europe (Catalá et al., 2015; Corcobado et al., 2023; Green et al., 2020; Jung, 2009; Jung et al., 1996, 2000, 2016, 2019; La Spada et al., 2022; Moralejo et al., 2009; Mora-Sala et al., 2022; Vannini et al., 2013; Vettraino et al., 2002). Since planting of infested nursery stock is the primary pathway of Phytophthora pathogens to the wider environment (Brasier et al., 2022; Frankel et al., 2020; Jung et al., 2016, 2018a; Pérez-Sierra & Jung, 2013) this study was focused on forest and ornamental nurseries in Czech Republic.
Using both traditional and metagenomic approaches, in total 50 Phytophthora taxa and many taxa from other oomycete genera were found in soil and water samples in seven of the eight nurseries tested. Many of the known Phytophthora and other oomycete taxa detected in this study, including the wide-host range pathogens P. cactorum, P. cryptogea, P. multivora, P. plurivora, and P. ×cambivora, were expected as they had been reported previously in Czech Republic and many European countries. For example, P. ×cambivora has been known to occur in the Czech Republic since 2008 (Černý et al., 2008). While Redondo et al. (2018) reported this species to be more common in nurseries than forests in Sweden, where the pathogen prefers managed (anthropogenic) forests, it is one of the main drivers of the devastating oak, beech and chestnut declines in Austria, Germany, Italy and other European countries (Cech & Jung, 2005; Corcobado et al., 2020; Jung, 2009; Jung et al., 2000, 2013a, 2018a, 2019; Milenković et al., 2012; Schmitz et al., 2009; Stępniewska & Dłuszyński, 2010; Telfer et al., 2015; Vettraino et al., 2002, 2005). Due to its presence in Czech nurseries, outbreaks of the diseases caused by P. ×cambivora can be expected also in the Czech Republic. Also P. plurivora, a wide-host range pathogen involved in European oak and beech declines with a widespread distribution in European nurseries (Corcobado et al., 2020, 2023; Jung et al., 2016, 2018a, 2019, 2024; Jung & Burgess, 2009; Mora-Sala et al., 2022), has previously been reported from Czech Republic (Mrázková et al., 2011, 2013). Phytophthora cinnamomi, arguably the most dangerous invasive Phytophthora pathogen with a global host range of more than 5000 plant species (Hardham & Blackman, 2018), was very prominent in our NGS study. It has occasionally been reported in the Czech Republic, mostly from Rhododendron and Vaccinium in ornamental nurseries and gardens (Černý et al., 2011, 2020; Mrázková et al., 2011). Common occurrence of the pathogen in irrigation water is probably a consequence of these records especially when filtration of contaminated water is not applied or not fully functional in the nurseries. In this study, P. cinnamomi was detected in both, non-filtered and filtered water. Model calculations indicate that ongoing climate changes will enable this pathogen to increase its activity in Central Europe (Burgess et al., 2017). This is also supported by recent outbreaks of P. cinnamomi in chestnut stands and blueberry plantations in Germany (Nechwatal & Jung, 2021; Peters et al., 2019).
However, the finding of the new species P. transitoria (Chen et al., 2022) and ITS1 DNA barcodes consistent with 15 novel Phytophthora taxa, five novel downy mildew species from the genera Hyaloperonospora and Plasmopara and several Phytophthora taxa with unknown, uncommon or geographically limited occurrence in Europe, i.e. P. lateralis, P. virginiana, P. sansomeana, P. kwongonina, P. mississippiae, P. gibbosa/P. gregata and P. taxon sulawesiensis, was unexpected. This study constitutes the first report of P. lateralis in the Czech Republic, albeit based only on DNA barcode data. The species was detected with the second highest number of reads in NGS in four of the five tested plant species and water samples from nursery no. 3. The dominant hosts of P. lateralis, Chamaecyparis spp. and other Cupressaceae, are popular ornamentals in the Czech Republic and elsewhere in Europe. The pathogen was first reported in Europe from a nursery in France in 1999 where it was reported as having been eradicated (Hansen et al., 1999). However, ten years later severe outbreaks of root and collar rot and aerial infections of Chamaecyparis trees occurred in Brittany, France, the Netherlands and the UK (EPPO, 2011; Green et al., 2013; Jung et al., 2018a; Robin et al., 2011). The distribution of P. lateralis in the rest of Europe is unknown. The explanation for the absence of P. lateralis in our soil baiting tests may be the intolerance of the pathogen to the generally hot and dry continental climatic conditions in the summer. Phytophthora virginiana and several closely related taxa were detected in rivers and waterways in Portugal (Jung et al., 2023, unpublished data). The species was probably introduced to Europe from Asia, where it is widespread. Phytophthora virginiana was involved in hybridisation processes resulting in several hybrids with P. virginiana as one parent (Jung et al., 2017a, 2020). This species is classified as tolerant to high temperatures (Yang & Hong, 2013) which may provide an advantage for the summer temperatures in Czech Republic. Phytophthora sansomeana was reported previously from Europe in Croatia and Germany (Jung et al., 2016). Grígel et al. (2019) detected P. sansomeana also in a fruit orchard in Czech Republic. The species P. gibbosa and P. gregata are sister taxa of subclade 6b described from Western Australia (Jung et al., 2011). They cannot be discriminated using the ITS1 sequences but to date P. gibbosa has never been found outside Western Australia whereas P. gregata has been reported from the Czech Republic by Černý et al. (2011) and Grígel et al. (2019). Known only from waterbodies in the USA, P. mississippiae is closely related to P. ornamentata described by Scanu et al. (2015) in Sardinia. Similarly, P. kwongonina which was first reported from Australia (Burgess et al., 2018) is closely related to P. rosacearum that was already reported from the Czech Republic by Grígel et al. (2019). Both species are closely related and have identical or highly similar ITS1 sequences, hence they cannot be distinguished (Burgess et al., 2017) by the ITS1 barcode used in this study. The informally named P. taxon sulawesiensis from Clade 6 was identified in only one soil sample from Chamaecyparis in this study. This species was abundantly detected in Indonesia and Japan (T. Jung, M. Horta Jung and I. Milenković, unpublished results) but it has not yet been detected in the Czech Republic or elsewhere in Europe. The presence of P. capsici or P. glovera, which also cannot be discriminated using the ITS1 barcode, was surprising. They are soilborne pathogens associated with vegetable crops, mainly Capsicum spp. and tobacco (Jung et al., 2024). Although not reported in forest nurseries, the ITS1 barcode of P. capsici/P. glovera has been reported from UK forests (Landa et al., 2021) and it cannot be excluded that an unknown sister taxon of P. capsici and P. glovera is occuring in European habitats.
The records of both known and novel non-native Phytophthora species in Czech nurseries in this study further illustrate problems of European plant biosecurity (Brasier, 2008; Jung et al., 2016, 2018a, 2024). The European plant biosecurity system primarily relies on visual inspection of plants for the presence of symptoms caused by listed quarantine organisms. However, the widespread use of fungicides and fungistatic chemicals in nurseries suppresses the development of disease symptoms making the detection of asymptomatic pathogens by visual inspections impossible (Brasier, 2008; Jung et al., 2016; Jung & Blaschke, 2004; Pérez-Sierra & Jung, 2013). Nursery no. 3 imports most of its plants from foreign nurseries and the consequences of this practice are manifested by a high diversity of Phytophthora spp. identified in this study. Other nursery surveys demonstrated Phytophthora presence in visually healthy trees with EU plant passports obtained from producers in Netherlands or Germany (Jung et al., 2016; Rossmann et al., 2021). Husson et al. (2007) also demonstrated that plants infected by P. ramorum were imported to France from the Netherlands while Ginetti et al. (2015) reported P. pachypleura from Aucuba japonica plants imported by Italian nurseries from France. The potential solution of these problems could involve regulation of pathogen routes and employing risk-based inspection procedures carried out by a sufficient number of trained personnel using advanced molecular detection techniques (Jung et al., 2016). These protocols are recommended for implementation both at entry points to countries and in nurseries to decrease the risks associated with the introduction and spread of both known and unknown potential pathogens to Europe (Jung et al., 2016).
DNA metabarcoding is an increasingly popular technique in current microbial diversity research (Taberlet et al., 2012) and also used in freshwater habitats (Matsuoka et al., 2022). In this study, the spectrum of Phytophthora spp. detected by both baiting and barcoding techniques differed considerably which is in common to other similar studies (Catalá et al., 2015; Bose et al., 2018; Riddell et al., 2019; Landa et al., 2021; Sarker et al., 2023a). Sarker et al. (2023a) and Burgess et al. (2017) mention that metabarcoding typically reveals a higher diversity of species in an environmental sample compared to that identified by isolation methods. This difference was also confirmed in this study. This is certainly caused by the fact that species with low levels of inoculum are outcompeted by those with higher levels. In addition, the environmental conditions during baiting are likely to bias the detection rate of all species present (Redekar et al., 2019). This may explain why there were generally fewer species detected by soil and water baiting than by DNA metabarcoding. However, occasionally baiting tests reveal the presence of Phytophthora taxa not detected with metagenomic approaches (La Spada et al., 2022; Sarker et al., 2023a). In this study, P. plurivora was the most common Phytophthora species isolated from soil samples by baiting but was not detected by metabarcoding of soil. Also P. cactorum, P. lacustris and P. syringae were not detected by DNA metabarcoding. There is no single clear explanation for this discrepancy but uneven scattered distribution of inoculum in soil in combination with the – compared to baiting – low volume of soil used for metabarcoding is a likely explanation. Ruiz Gómez et al., 2019 detected P. plurivora as the most common Phytophthora species in holm oak stands in Andalusia (Spain) using a similar NGS method. Also, Rossmann et al. (2021) detected P. plurivora in the soil of nursery plants using the same methodology of DNA extraction and DNA metabarcoding. Therefore, the failure to detect P. plurivora using NGS in our study is probably not caused by any methodological failing such as PCR primer specificity. In this study, the absence of P. lacustris in the DNA barcoding data from water samples was particularly unexpected given its frequent detection with baits and the reported detection of this species in similar NGS studies (Catalá et al., 2015; Landa et al., 2021). It appears that in our metabarcoding test competition within the PCR with other highly abundant taxa such as P. cinnamomi and P. lateralis may have outcompeted amplifcication of P. lacustris barcodes in the water samples.
Phytophthora species with resting spores (oospores, chlamydospores) that germinate slowly or remain dormant are difficult to isolate using baiting tests, but their DNA is available for detection using metabarcoding (Sarker et al., 2023a). However, the detection of eDNA by metabarcoding confirms the presence of an organism but its viability remains unclear since DNA of a dead organism can also be detected (Burgess et al., 2017). In contrast to metabarcoding approaches, traditional isolation methods produce living isolates confirming that an organism is alive and enabling further studies on the taxonomy, ecology, pathogenicity and control of Phytophthora pathogens (Sarker et al., 2023a). Overall, although baiting is a slower and less effective method, it is still necessary to use it together with NGS methods due to the potential detection of taxa not found with NGS, the availability of living isolates for further studies and the knowledge that detected taxa are alive.
Water sourced from rivers, reservoirs and ponds can pose a substantial threat for pathogen dispersal to nurseries. (Pettitt, 2017). Numerous studies have demonstrated an ubiquitous high diversity of Phytophthora species and other oomycetes in rivers and streams in Australia, the Americas, Asia, Africa and Europe (e.g. Caballol et al., 2024; Catalá et al., 2015; Corcobado et al., 2023; Hüberli et al., 2013; Jung et al., 2017a, 2018b, 2019, 2020, 2022, 2024; Oh et al., 2013; Reeser et al., 2011; Seddaiu et al., 2020; Shrestha et al., 2013). Previous research confirmed the presence of Phytophthora species in nursery irrigation water using baiting tests. Themann et al. (2002) found a wide range of Phytophthora species in recirculation systems of ornamental nurseries in Germany. Rytkonen et al. (2008) reported detection of P. cactorum in Finnish nurseries by baiting with Rhododendron leaves. Mrázková et al. (2011) detected P. gonapodyides by baiting from irrigation water in an ornamental nursery of Czech Republic. However, single sampling by baiting cannot reveal the total diversity of oomycetes. In metagenomic approaches, filtering of water samples is often the first step before the DNA extraction and since it can allow a larger volume of water to be examined than from baiting it has been shown to enable detection of a wider range of species diversity (Marčiulynas et al., 2020, Rodríguez-Padrón et al., 2019, Matsuoka et al., 2022).
Slow sand filters appear to be a promising method for decreasing the numbers of pathogen propagules in irrigation water (Kubiak et al., 2015; Ufer et al., 2008). However, our results suggested that in the tested nursery the filtration of water using sand filters does not eliminate oomycetes suficiently from infested irrigation water. Interestingly, we detected higher diversity of oomycete species (27 species) in the water after filtration than in the river (9 species) which is the source of the irrigation water. Although there were four samples taken after filtration and only one sample taken from the river, at least 15 species were detected in each of the filtered water samples. Therefore, the efficiency of these filters to prevent dispersal of Phytophthora pathogens is questionable. More detailed investigations are required but it seems probable that the sand filters in this nursery contain a population of Phytophthora propagules and are not sufficient for removing Phytophthora spores from the water. Because surplus irrigation water is recycled and reused after watering, the greater diversity of pathogens in the nursery irrigation system is not entirely surprising. In agriculture and horticulture, slow sand filters are used commonly to efficiently eliminate pathogen propagules from water more economically in comparison to other techniques (e.g. ozonation, thermal and chemical treatments or UV radiation) (Runia et al., 1997; Ufer et al., 2008; Van Os et al., 2000). The crucial factor for the functionality of slow sand filters is, however, the cleaning and regular maintenance of these filters. Therefore, it seems possible that the staff of the respective nursery did not clean and maintain the filters as recommended by the scientific literature.
In this study, both methods (DNA metabarcoding and soil baiting) confirmed that the forest nursery (nursery no. 8) had much lower Phytophthora diversity (2 taxa) than the ornamental nursery (nursery no. 3; 32 taxa) which regularly imports a high volume of plant material. The import of a host species, the transport via transport vector, or natural expansion from adjacent regions are recognized as three principal ways of non-native species introduction (Hulme et al., 2008). Therefore, native or long-established introduced Phytophthora species are expected to be dominant in forest nurseries with local production of plant material whereas non-native Phytophthora species and sometimes even novel Phytophthora species are more likely to occur in ornamental nurseries with regular import of nursery stock. Liebhold et al. (2012) noted that ca 70% of problematic forest pests and pathogens occurring in the US between 1860 and 2006 are probably associated with imported live plants. Similarly, Jung et al. (2016) hypothesized that the import of living plants for planting from overseas is responsible for most, if not all, introductions of exotic Phytophthora species to Europe and suggested a pathway regulation approach and phytosanitary inspections based on modern high-throughput metagenomic approaches instead of visual inspections for potential symptoms of listed quarantine organisms.
Conclusion
This study demonstrates the widespread presence of 50 Phytophthora taxa and other oomycete genera in plant nursery stock in the Czech Republic. Only 24% of the detected Phytophthora species were recovered by baiting while 90% were detected by eDNA metabarcoding. Only 7 species were detected by both techniques but usually from different samples. Phytophthora cinnamomi and P. lateralis were particularly common as revealed by DNA metabarcoding but they were not obtained by baiting. Therefore, both tested detection methods complement each other. Some species can be more easily isolated by baiting, but they do not necessarily have to be the most abundant species. DNA metabarcoding revealed that an ornametal nursery with regular imports of plants had much higher diversity of Phytophthora (32 taxa) than a forest nursery with exclusively local production (2 taxa) highlighting the biosecurity risk posed by plant imports. Results revealed that water filtration using sand filters does not prevent the occurrence of oomycetes in irrigation water if these filters are not maintained properly. The results of metabarcoding revealed numerous unexpected and undescribed oomycete species. It is evident that in plant biosecurity the use of both traditional and modern metabarcoding techniques is necessary to detect all known and unknown pathogens.
Data availability
Data are available in a publicly accessible repository. The raw Illumina data presented in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.10000121 as FASTQ files.
References
Abad, Z. G., Burgess, T. I., Bourret, T., Bensch, K., Cacciola, S. O., Scanu, B., Mathew, R., Kasiborski, B., Srivastava, S., Kageyama, K., Bienapfl, J. C., Verkleij, G., Broders, K., Schena, L., & Redford, A. J. (2023). Phytophthora: Taxonomic and phylogenetic revision of the genus. Studies in Mycology, 106, 259–348. https://doi.org/10.3114/sim.2023.106.05
Aghighi, S., Hardy, G. E. S. J., Scott, J. K., & Burgess, T. I. (2012). Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. European Journal of Plant Pathology, 133, 841–855. https://doi.org/10.1007/s10658-012-0006-5
Balci, Y., & Halmschlager, E. (2003a). Incidence of Phytophthora species in oak forests in Austria and their possible involvement in oak decline. Forest Pathology, 33, 157–174. https://doi.org/10.1046/j.1439-0329.2003.00318.x
Balci, Y., & Halmschlager, E. (2003b). Phytophthora species in oak ecosystems in Turkey and their association with declining oak trees. Plant Pathology, 52, 694–702. https://doi.org/10.1111/j.1365-3059.2003.00919.x
Bose, T., Wingfield, M. J., Roux, J., Vivas, M., & Burgess, T. I. (2018). Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecology, 36, 17–25. https://doi.org/10.1016/j.funeco.2018.09.001
Brasier, C. M. (2008). The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology, 57, 792–808. https://doi.org/10.1111/j.1365-3059.2008.01886.x
Brasier, C. M., Robredo, F., & Ferraz, J. F. P. (1993). Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathology, 42, 140–145. https://doi.org/10.1111/j.1365-3059.1993.tb01482.x
Brasier, C. M., Kirk, S. A., Delcan, J., Cooke, D. E. L., Jung, T., & Man in’t Veld, W. A. (2004). Phytophthora alni sp. nov. and its variants: Designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycological Research, 2004(108), 1172–1184. https://doi.org/10.1017/S0953756204001005
Brasier, C. M., Beales, P. A., Kirk, S. A., Denman, S., & Rose, J. (2005). Phytophthora kernoviae sp. nov. an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in Britain. Mycological Research, 109, 853–859. https://doi.org/10.1017/S0953756205003357
Brasier, C. M., Vettraino, A. M., Chang, T. T., & Vannini, A. (2010). Phytophthora lateralis discovered in an old growth Chamaecyparis forest in Taiwan. Plant Pathology, 59, 595–603. https://doi.org/10.1111/j.1365-3059.2010.02278.x
Brasier, C. M., Franceschini, S., Vettraino, A., Hansen, E. M., Green, S., Robin, C., Webber, J. F., & Vannini, A. (2012). Four phenotypically and phylogenetically distinct lineages in Phytophthora lateralis. Fungal Biology, 116, 1232–1249. https://doi.org/10.1016/j.funbio.2012.10.002
Brasier, C. M., Scanu, B., Cooke, D. E. L., & Jung, T. (2022). Phytophthora: An ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus, 13, 12. https://doi.org/10.1186/s43008-022-00097-z
Burgess, T. I., Simamora, A. V., White, D., Wiliams, B., Schwager, M., Stukely, M. J. C., & Hardy, G. E. S. J. (2018). New species from Phytophthora clade 6a: Evidence for recent radiation. Persoonia, 41, 1–17. https://doi.org/10.3767/persoonia.2018.41.01
Burgess, T. I., White, D., McDougall, K. M., Garnas, J., Dunstan, W. A., Catala, S., Carnegie, A. J., Worboys, S., Cahill, D., Vettraino, A.-M., Stukely, M. J. C., Liew, E. C. Y., Paap, T., Bose, T., Migliorini, D., Williams, B., Brigg, F., Crane, C., Rudman, T., & Hardy, G. E. S. J. (2017). Distribution and diversity of Phytophthora across Australia. Pacific Conservation Biology, 2017(23), 150–162. https://doi.org/10.1071/PC16032
Bush, E. A., Hong, C. X., & Stromberg, E. L. (2003). Fluctuations of Phytophthora and Pythium spp. in components of a recycling irrigation system. Plant Disease, 87(12), 1500–1506. https://doi.org/10.1094/PDIS.2003.87.12.1500
Bush, E. A., Stromberg, E. L., Hong, C. X., Richardson, P. A., & Kong, P. (2006). Illustration of key morphological characteristics of Phytophthora species identified in Virginia nursery irrigation water. Plant Health Progress, 7(1). https://doi.org/10.1094/PHP-2006-0621-01-RS
Caballol, M., Redondo, M. A., Catalán, N., Corcobado, T., Jung, T., Marcais, B., Milenković, I., Nemesio-Gorriz, M., & Oliva, J. (2024). Climate acts as an environmental filter to plant pathogens. The ISME Journal, 18(1), wrae010. https://doi.org/10.1093/ismejo/wrae010
Cacciola, S. O., Motta, E., Raudino, F., Chimento, A., Pane, A., & San Lio, G. M. (2005). Phytophthora pseudosyringae the causal agent of bleeding cankers of beech in Central Italy. Journal of Plant Pathology, 87, 289.
Catalá, S., Pérez-Sierra, A., & Abad-Campos, P. (2015). The use of genus-specific amplicon pyrosequencing to assess Phytophthora species diversity using eDNA from soil and water in northern Spain. PLoS One, 10, e0119311. https://doi.org/10.1371/journal.pone.0119311
Cech, T. L., & Jung, T. (2005). Phytophthora—Wurzelhalsfäulen an Buchen nehmen Auch in Österreich zu (Phytophthora root rot of beech is also increasing in Austria). Forstschutz Aktuell, 34, 7.
Černý, K., Havrdová, L., Němec, P., Hrabětová, M., Mrázková, M., Zahradník, D., Grígel, J., & Šetinová, D. (2020). Integrovaná ochrana sazenic v lesních školkách před patogeny z r. Phytophthora. Certified methodics 68354/2020-MZE-16222/M216. Výzkumný ústav pro krajinu a okrasné zahradnictví.
Černý, K., Tomšovský, M., Mrázková, M., & Strnadová, V. (2011). The present state of knowledge on Phytophthora spp. diversity in forest and ornamental woody plants in the Czech Republic. New Zealand Journal of Forestry Science, 41, 75–82.
Černý, K., Gregorová, B., Strnadová, B., Tomšovský, M., Holub, V., & Gabrielová, Š. (2008). Phytophthora cambivora causing ink disease of sweet chestnut recorded in the Czech Republic. Czech mycology, 60, 267–276. https://doi.org/10.33585/cmy.60210.
Chen, Q., Bakhshi, M., Balci, Y., Broders, K. D., Cheewangkoon, R., Chen, S. F., Fan, X. L., Gramaje, D., Halleen, F., Horta Jung, M., Jiang, N., Jung, T., Májek, T., Marincowitz, S., Milenković, I., Mostert, L., Nakashima, C., Nurul Faziha, I., Pan, M., et al. (2022). Genera of phytopathogenic fungi: GOPHY 4. Studies in Mycology, (101), 417–564. https://doi.org/10.3114/sim.2022.101.06
Cock, P. J. A., Cooke, D. E. L., Thorpe, P., & Pritchard, L. (2023). THAPBI PICT-a fast, cautious, and accurate metabarcoding analysis pipeline. PeerJ, 11, e15648. https://doi.org/10.7717/peerj.15648
Cooke, D. E. L., Drenth, A., Duncan, J. M., Wagels, G., & Brasier, C. M. (2000). A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology, 30, 17–32. https://doi.org/10.1006/fgbi.2000.1202
Corcobado, T., Cech, T. L., Daxer, A., Ďatková, H., Janoušek, J., Patra, S., Jahn, D., Hüttler, C., Milenković, I., Tomšovský, M., Horta Jung, M., & Jung, T. (2023). Phytophthora, Nothophytophthora and Halophytophthora diversity in rivers, streams and riparian alder ecosystems of Central Europe. Mycological Progress, 22, 50. https://doi.org/10.1007/s11557-023-01898-1
Corcobado, T., Cech, T. L., Brandstetter, M., Daxer, A., Hüttler, C., Kudláček, T., Horta Jung, M., & Jung, T. (2020). Decline of European beech in Austria: Involvement of Phytophthora spp. and contributing biotic and abiotic factors. Forests, 11, 895. https://doi.org/10.3390/f11080895
Day, W. R. (1938). Root-rot of sweet chestnut and beech caused by species of Phytophthora. I. Cause and symptoms of disease: Its relation to soil conditions. Forestry, 12, 101–116. https://doi.org/10.1093/oxfordjournals.forestry.a062747
Denman, S., Kirk, S. A., Moralejo, E., & Webber, J. F. (2009). Phytophthora ramorum and Phytophthora kernoviae on naturally infected asymptomatic foliage. Bulletin OEPP/EPPO Bulletin, 39, 105–111. https://doi.org/10.1111/j.1365-2338.2009.02243.x
EPPO. (2011). Phytophthora lateralis detected again in the Netherlands (p. 28). EPPO Reporting Service no. 02.
Erwin, D. C., & Ribeiro, O. K. (1996). Phytophthora diseases worldwide (p. 562). The American Phytopathological Society.
Frankel, S. J., Conforti, C., Hillman, J., Ingolia, M., Shor, A., Benner, D., Alexander, J. M., Bernhardt, E., & Swiecki, T. J. (2020). Phytophthora introductions in restoration areas: Responding to protect California native flora from human-assisted pathogen spread. Forests, 11, 1291. https://doi.org/10.3390/f11121291
Ginetti, B., Ragazzi, A., Carmignani, S., & Moricca, S. (2015). Collar rot and crown wilting by Phytophthora pachypleura on Aucuba japonica in Italian nurseries. Plant Disease, 99, 860. https://doi.org/10.1094/PDIS-01-15-0120-PDN
Green, S., Brasier, C. M., Schlenzig, A., McCracken, A., MacAskill, G. A., Wilson, M., & Webber, J. F. (2013). The destructive invasive pathogen Phytophthora lateralis found on Chamaecyparis lawsoniana across the UK. Forest Pathology, 43, 19–28. https://doi.org/10.1111/j.1439-0329.2012.00788.x
Green, S., Riddell, C. E., Frederickson-Matika, D., Armstrong, A., Elliot, M., Forster, J., Hedley, P. E., Morris, J., Thorpe, P., Cooke, D. E., Sharp, P. M., & Pritchard, L. (2020). Diversity of woody-host infecting Phytophthora species in public parks and botanic gardens as revealed by metabarcoding, and opportunities for mitigation through best practice. Sibbaldia: The International Journal of Botanic Garden Horticulture, 18, 67–88. https://doi.org/10.24823/Sibbaldia.2020.289
Greslebin, A. G., Hansen, E. M., & Sutton, W. (2007). Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentina). Mycological Research, 111, 308–316. https://doi.org/10.1016/j.mycres.2007.01.008
Grígel, J., Černý, K., Mrázková, M., Havrdová, L., Zahradník, D., Jílková, B., & Hrabětová, M. (2019). Phytophthora root and collar rots in fruit orchards in the Czech Republic. Phytopathologia Mediterranea, 58(2), 261–275. https://doi.org/10.14601/PhytopatholMediter-10614
Hansen, E. M. (2011). Phytophthora lateralis. Forest Phytophthoras, 1. https://doi.org/10.5399/osu/fp.1.1.1816
Hansen, E. M., Streito, J. C., & Delatour, C. (1999). First confirmation of Phytophthora lateralis in Europe. Plant Disease, 83, 587. https://doi.org/10.1094/PDIS.1999.83.6.587B
Hansen, E. M., Goheen, D. J., Jules, E. S., & Ullian, B. (2000). Managing port-Orford-cedar and the introduced pathogen Phytophthora lateralis. Plant Disease, 84, 4–14. https://doi.org/10.1094/PDIS.2000.84.1.4
Hansen, E. M., Reeser, P. W., & Sutton, W. (2012). Phytophthora beyond agriculture. Annual Reviews in Phytopathology, 50, 359–378. https://doi.org/10.1146/annurev-phyto-081211-172946
Hardham, A. R. (2001). The cell biology behind Phytophthora pathogenicity. Australasian Plant Pathology, 30, 91–98. https://doi.org/10.1071/AP01006
Hardham, A. R., & Blackman, L. M. (2018). Phytophthora cinnamomi. Molecular Plant Pathology, 19, 260–285. https://doi.org/10.1111/mpp.12568
Ho, H. H. (2018). The taxonomy and biology of Phytophthora and Pythium. Journal of Bacteriology and Mycology Open Access, 00174, 6(2). https://doi.org/10.15406/jbmoa.2018.06.00174
Hüberli, D., Hardy, G. E. S. J., White, D., Williams, N., & Burgess, T. I. (2013). Fishing for Phytophthora from Western Australia’s waterways: A distribution and diversity survey. Australasian Plant Pathology, 42, 251–260. https://doi.org/10.1007/s13313-012-0195-6
Hulme, P. E., Bacher, S., Kenis, M., Klotz, S., Kühn, J., Minchin, D., Nentwig, W., Olenin, S., Panov, V., Pergl, J., Pyšek, P., Roques, A., Sol, D., Solarz, W., & Vilà, M. (2008). Grasping at the routes of biological invasions: A framework for integrating pathways into policy. Journal of Applied Ecology, 45, 403–414. https://doi.org/10.1111/j.1365-2664.2007.01442.x.
Husson, C., Delatour, C., Frey, P., Marcais, B., Saurat, C., & Schenck, N. (2007). First report of Phytophthora ramorum on ornamental plants in France. Plant Disease, 91(10), 1359–1359. https://doi.org/10.1094/PDIS-91-10-1359B
Illumina Inc. (2013). 16S Metagenomic Sequencing Library Preparation—Preparing 16S ribosomal RNA gene amplicons for the illumina MiSeq system. Illumina Inc.
Jung, T., Blaschke, H., & Neumann, P. (1996). Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. European Journal of Forest Pathology, 26, 253–272. https://doi.org/10.1111/j.1439-0329.1996.tb00846.x
Jung, T., Blaschke, H., & Oßwald, W. (2000). Involvement of soilborne Phytophthora species in central European oak decline and the effect of site factors on the disease. Plant Pathology, 49, 706–718. https://doi.org/10.1046/j.1365-3059.2000.00521.x
Jung, T., & Blaschke, M. (2004). Phytophthora root and collar rot of alders in Bavaria: Distribution, modes of spread, and possible management strategies. Plant Pathology, 53, 197–208. https://doi.org/10.1111/j.0032-0862.2004.00957.x
Jung, T., Hudler, G. W., Jensen-Tracy, S. L., Griffiths, H. M., Fleischmann, F., & Oßwald, W. (2005). Involvement of Phytophthora species in the decline of European beech in Europe and the USA. Mycologist, 19(4), 159–166. https://doi.org/10.1017/S0269-915X(05)00405-2
Jung, T. (2009). Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. Forest Pathology, 39, 73–94. https://doi.org/10.1111/j.1439-0329.2008.00566.x
Jung, T., & Burgess, T. I. (2009). Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia, 22, 95–110. https://doi.org/10.3767/003158509X442612
Jung, T., Stukely, M. J. C., Hardy, G. E. S. J., White, D., Paap, T., Dunstan, W. A., & Burgess, T. I. (2011). Multiple new Phytophthora species from ITS clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia, 26, 13–39. https://doi.org/10.3767/003158511X557577
Jung, T., Vettraino, A. M., Cech, T., & Vannini, A. (2013a). The impact of invasive Phytophthora species on European forests. In K. Lamour (Ed.), Phytophthora: A global perspective (pp. 146–158). CABI. https://doi.org/10.1079/9781780640938.0146
Jung, T., Colquhoun, I. J., & Hardy, G. E. S. J. (2013b). New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. Forest Pathology, 43, 266–288. https://doi.org/10.1111/efp.12025
Jung, T., Orlikowski, L., Henricot, B., Abad-Campos, P., Aday, A. G., Aguín Casal, O., Bakonyi, J., Cacciola, S. O., Cech, T., Chavarriaga, D., Corcobado, T., Cravador, A., Decourcelle, T., Denton, G., Diamandis, S., Dogmus-Lehtijärvi, H. T., Franceschini, A., Ginetti, B., Glavendekić, M., et al. (2016). Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathology, 46, 134–163. https://doi.org/10.1111/efp.12239
Jung, T., Chang, T.-T., Bakonyi, J., Seress, D., Pérez-Sierra, A., Yang, X., Hong, C., Scanu, B., Fu, C. H., Hsueh, K.-L., Maia, C., Abad-Campos, P., Léon, M., & Horta Jung, M. (2017a). Diversity of Phytophthora species in natural ecosystems of Taiwan and association with disease symptoms. Plant Pathology, 66, 194–211. https://doi.org/10.1111/ppa.12564
Jung, T., Jung, M. H., Cacciola, S. O., Cech, T., Bakonyi, J., Seress, D., Mosca, S., Schena, L., Seddaiu, S., Pane, A., San Lio, G. M., Maia, C., Cravador, A., Franceschini, A., & Scanu, B. (2017b). Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus, 8, 219–244. https://doi.org/10.5598/imafungus.2017.08.02.02
Jung, T., Scanu, B., Bakonyi, J., Seress, D., Kovács, G. M., Durán, A., von Stowasser, E. S., Schena, L., Mosca, S., Thu, P. Q., Nguyen, C. M., Fajardo, S., González, M., Pérez-Sierra, A., Rees, H., Cravador, A., Maia, C., & Horta Jung, M. (2017c). Nothophytophthora gen. nov., a new sister genus of Phytophthora from natural and semi-natural ecosystems. Persoonia, 39, 143–174. https://doi.org/10.3767/persoonia.2017.39.07
Jung, T., Pérez-Sierra, A., Durán, A., Horta Jung, M., Balci, Y., & Scanu, B. (2018a). Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia, 40, 182–220. https://doi.org/10.3767/persoonia.2017.39.07
Jung, T., Durán, A., von Stowasser, E. S., Schena, L., Mosca, S., Fajardo, S., González, M., Navarro Ortega, A. D., Bakonyi, J., Seress, D., Tomšovský, M., Cravador, A., Maia, C., & Horta Jung, M. (2018b). Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. Forest Pathology, 48, e12443. https://doi.org/10.1111/efp.12443
Jung, T., La Spada, F., Pane, A., Aloi, F., Evoli, M., Horta Jung, M., Scanu, B., Faedda, R., Rizza, C., Puglisi, I., San Lio, G. M., Schena, L., & Cacciola, S. O. (2019). Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests, 10, 259. https://doi.org/10.3390/f10030259
Jung, T., Scanu, B., Brasier, C. M., Webber, J., Milenković, I., Corcobado, T., Tomšovský, T., Pánek, M., Bakonyi, J., Maia, C., Bačová, A., Raco, M., Rees, H., Pérez-Sierra, A., & Horta Jung, M. (2020). A survey in natural forest ecosystems of Vietnam reveals high diversity of both new and described Phytophthora taxa including P. ramorum. Forests, 11, 93. https://doi.org/10.3390/f11010093
Jung, T., Horta Jung, M., Webber, J. F., Kageyama, K., Hieno, A., Masuya, H., Uematsu, S., Pérez-Sierra, A., Harris, A. R., Forster, J., Rees, H., Scanu, B., Patra, S., Kudláček, T., Janoušek, J., Corcobado, T., Milenković, I., Nagy, Z., Csorba, I., et al. (2021). The destructive tree pathogen Phytophthora ramorum originates from the Laurosilva forests of East Asia. Journal of Fungi, 7, 226. https://doi.org/10.3390/jof7030226
Jung, T., Milenković, I., Corcobado, T., Májek, T., Janoušek, J., Kudláček, T., Tomšovský, M., Nagy, Z., Durán, A., Tarigan, M., Sanfuentes von Stowasser, E., Singh, R., Ferreira, M., Webber, J., Scanu, B., Chi, N. M., Thu, P. Q., Junaid, M., Rosmana, A., et al. (2022). Extensive morphological and behavioural diversity among fourteen new and seven described species in Phytophthora clade 10 and its evolutionary implications. Persoonia, 49, 1–57. https://doi.org/10.3767/persoonia.2022.49.01
Jung, T., Balci, Y., Broders, K. D., Milenković, I., Janoušek, J., Kudláček, T., Đorđević, B., & Horta Jung, M. (2023). Synchrospora gen. nov., a new Peronosporaceae genus with aerial lifestyle from a natural cloud forest in Panama. Journal of Fungi, 517. https://doi.org/10.3390/jof9050517
Jung, T., Milenković, I., Balci, Y., Janoušek, J., Kudláček, T., Nagy, Z. Á., Baharuddin, B., Bakonyi, J., Broders, K. D., Cacciola, S. O., et al. (2024). Worldwide forest surveys reveal forty-three new species in Phytophthora major clade 2 with fundamental implications for the evolution and biogeography of the genus and global plant biosecurity. Studies in Mycology, 107, 251–388. https://doi.org/10.3114/sim.2024.107.04
Khew, K. L., & Zentmyer, G. A. (1973). Chemotactic response of zoospores of five species of Phytophthora. Phytopathology, 63, 1511–1517. https://doi.org/10.1094/Phyto-63-1511
Kroon, L. P. N. M., Bakker, F. T., van den Bosch, G. B. M., Bonants, P. J. M., & Flier, W. G. (2004). Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology, 41, 766–782. https://doi.org/10.1016/j.fgb.2004.03.007
Kubiak, K., Błaszczyk, M., Sierota, Z., Tkaczyk, M., & Oszako, T. (2015). Slow sand filtration for elimination of phytopathogens in water used in forest nurseries. Scandinavian Journal of Forest Research, 30, 664–577. https://doi.org/10.1080/02827581.2015.1048716
Lamour, K., & Finley, L. (2006). A strategy for recovering high quality genomic DNA from a large number of Phytophthora isolates. Mycologia, 98, 514–517. https://doi.org/10.3852/mycologia.98.3.514
Landa, B. B., Arias-Giraldo, L. F., Henricot, B., Montes-Borrego, M., Shuttleworth, L. A., & Pérez-Sierra, A. (2021). Diversity of Phytophthora species detected in disturbed and undisturbed British soils using high-throughput sequencing targeting ITS rRNA and COI mtDNA regions. Forests, 12, 229. https://doi.org/10.3390/f12020229
La Spada, F., Cock, P. J. A., Randall, E., Pane, A., Cooke, D. E. L., & Cacciola, S. O. (2022). DNA metabarcoding and isolation by baiting complement each other in revealing Phytophthora diversity in anthropized and natural ecosystems. Journal of Fungi, 8, 330. https://doi.org/10.3390/jof8040330
Liebhold, A. M., Brockerhoff, E. G., Garrett, L. J., Parke, J. L., & Britton, K. O. (2012). Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Frontiers in Ecology and Environments, 10, 135–143. https://doi.org/10.1890/110198
MacDonald, J. D., Ali-Shtayeh, M. S., Kabashima, J., & Stites, J. (1994). Occurrence of Phytophthora species in recirculated nursery irrigation effluents. Plant Disease, 78, 607–611. https://doi.org/10.1094/PD-78-0607
Marčiulynas, A., Marčiulynienė, D., Lynikienė, J., Gedminas, A., Vaičiukynė, M., & Menkis, A. (2020). Fungi and oomycetes in the irrigation water of forest nurseries. Forests, 11, 459. https://doi.org/10.3390/f11040459
Martin, F. N., & Tooley, P. W. (2003). Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia, 95, 269–284. https://doi.org/10.1080/15572536.2004.11833112
Matsuoka, S., Sugiyama, Y., Nagano, M., & Doi, H. (2022). Influence of DNA extraction kits on freshwater fungal DNA metabarcoding. PeerJ, 10, e13477. https://doi.org/10.7717/peerj.13477
Maxwell, A., Vettraino, A., Eschen, R., & Andjic, V. (2024). International plant trade and biosecurity. In G. Dixon & D. Aldous (Eds.), Horticulture: Plants for people and places, volume 3. Springer. https://doi.org/10.1007/978-94-017-8560-0_9
Mendoza, M. L. Z., Sicheritz-Pontén, T., & Gilbert, M. T. P. (2015). Environmental genes and genomes: Understanding the differences and challenges in the approaches and software for their analyses. Briefings in Bioinformatics, 16, 745–758. https://doi.org/10.1093/bib/bbv001
Migliorini, D., Ghelardini, L., Tondini, E., Luchi, N., & Santini, A. (2015). The potential of symptomless potted plants for carrying invasive soilborne plant pathogens. Diversity and Distributions, 21, 1218–1229. https://doi.org/10.1111/ddi.12347
Milenković, I., Keča, N., Karadžić, D., Nowakowska, J. A., Borys, M., Sikora, K., & Oszako, T. (2012). Incidence of Phytophthora species in beech stands in Serbia. Folia Forestalia Polonica, Series A, 54(4), 223–232 https://zenodo.org/records/30775
Mrázková, M., Černý, K., Tomšovský, M., & Strnadová, V. (2011). Phytophthora plurivora T. Jung & T. I. Burgess and other Phytophthora species causing important diseases of ericaceous plants in the Czech Republic. Plant Protection Science, 47, 13–19. https://doi.org/10.17221/3108-PPS
Mrázková, M., Černý, K., Tomšovský, M., Strnadová, V., Gregorová, B., Holub, V., Pánek, M., Havrdová, L., & Hejná, M. (2013). Occurrence of Phytophthora multivora and Phytophthora plurivora in the Czech Republic. Plant Protection Science, 49, 155–164. https://doi.org/10.17221/74/2012-PPS
Moralejo, E., Pérez-Sierra, A., Álvarez, L. A., Belbahri, L., Lefort, F., & Descals, E. (2009). Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain. Plant Pathology, 58, 100–110. https://doi.org/10.1111/j.1365-3059.2008.01930.x
Mora-Sala, B., León, M., Pérez-Sierra, A., & Abad-Campos, P. (2022). New reports of Phytophthora species in plant nurseries in Spain. Pathogens, 11, 826. https://doi.org/10.3390/pathogens11080826
Nechwatal, J., & Jung, T. (2021). A study of Phytophthora spp. in declining highbush blueberry in Germany reveals that P. cinnamomi can thrive under central European outdoor conditions. Journal of Plant Diseases and Protection, 128, 769–774. https://doi.org/10.1007/s41348-021-00445-y
Oh, E., & Hansen, E. A. (2007). Histopathology of infection and colonization of susceptible and resistant port-Orford-cedar by Phytophthora lateralis. Phytopathology, 97, 684–693. https://doi.org/10.1094/PHYTO-97-6-0684
Oh, E., Gryzenhout, M., Wingfield, B. D., Wingfield, M. J., & Burgess, T. I. (2013). Surveys of soil and water reveal a goldmine of Phytophthora diversity in south African natural ecosystems. IMA Fungus, 4, 123–131. https://doi.org/10.5598/imafungus.2013.04.01.12
Orlikowski, L. B., Ptaszek, M., Trzewik, A., Orlikowska, T., & Wojtkowska, M. (2010). Occurrence of Phytophthora spp. in water and pathogenicity of chosen isolates of Phytophthora citricola for plants (Polish with English Abstract). Zeszyty Problemowe Postępy Nauk Rolniczych, 554, 155–160.
Orlikowski, L. B., Ptaszek, M., Trzewik, A., & Orlikowska, T. (2011). Usefulness of plant leaf baits for detection of Phytophthora spp. from water (Polish with English Abstract). Sylwan, 155, 493–499.
Orlikowski, L. B., Meszka, B., Ptaszek, M., Trzewik, A., & Orlikowska, T. (2012). A threat to the environment and horticultural plants posed by new Phytophthora species isolated from water (Polish with English Abstract). Woda-Środowisko-Obszary Wiejskie, 12, 171–178.
Oßwald, W., Fleischmann, F., Rigling, D., Coelho, A. C., Cravador, A., Diez, J., Dalio, R. J., Horta Jung, M., Pfanz, H., Robin, C., Sipos, G., Solla, A., Cech, T., Chambery, A., Diamandis, S., Hansen, E., Jung, T., Orlikowski, L. B., Parke, J., et al. (2014). Strategies of attack and defence in woody plant-Phytophthora interactions. Forest Pathology, 44(3), 169–190. https://doi.org/10.1111/efp.12096
Parke, J. L., Knaus, B. J., Fieland, V. J., Lewis, C., & Grünwald, N. J. (2014). Phytophthora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology, 104, 1052–1062. https://doi.org/10.1094/PHYTO-01-14-0014-R
Pérez-Sierra, A., & Jung, T. (2013). Phytophthora in woody ornamental nurseries. In K. Lamour (Ed.), Phytophthora: A global perspective: CABI (pp. 166–177). Wallingford. https://doi.org/10.1079/9781780640938.0166
Pérez-Sierra, A., Horta Jung, M., & Jung, T. (2022). Survey and monitoring of Phytophthora species in natural ecosystems: Methods for sampling, isolation, purification, storage, and pathogenicity tests. In N. Luchi (Ed.), Plant pathology. Methods in molecular biology, 2536 (pp. 23–49). Humana. https://doi.org/10.1007/978-1-0716-2517-0_2
Perrings, C., Dehnen-Schmutz, K., Touza, J., & Williamson, M. (2005). How to manage biological invasions under globalization. Trends in Ecology and Evolution, 20, 212–215. https://doi.org/10.1016/j.tree.2005.02.011
Peters, F. S., Wunderlich, F., & Metzler, B. (2019). First report of Phytophthora cinnamomi in forest stands in Germany. Forest Pathology, 49, e12485. https://doi.org/10.1111/efp.12485
Pettitt, T. R. (2017). Irrigation water and the health of nursery crops. In: C. Hong, G. W. Moorman, W. Wohanka, C. Büttner (Eds.), Biology, detection, and management of plant pathogens in irrigation water (pp. 13–22). The American Phytopathological Society. https://doi.org/10.1094/9780890544914.003
Pimentel, D., Zuniga, R., & Morrison, D. (2005). Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics, 52, 273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
Prospero, S., Vercauteren, A., Heungens, K., Belbahri, L., & Rigling, D. (2013). Phytophthora diversity and the population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathology, 62(5), 1063–1071. https://doi.org/10.1111/ppa.12027
Redekar, N. R., Eberhart, J. L., & Parke, J. L. (2019). Diversity of Phytophthora, Pythium, and Phytopythium species in recycled irrigation water in a container nursery. Phytobiomes Journal, 3(1), 2471–2906. https://doi.org/10.1094/PBIOMES-10-18-0043-R
Redondo, M. A., Boberg, J., Stenlid, J., & Oliva, J. (2018). Functional traits associated with the establishment of introduced Phytophthora spp. in Swedish forests. Journal of Applied Ecology, 55, 1538–1552. https://doi.org/10.1111/1365-2664.13068
Reeser, P. W., Sutton, W., Hansen, E. M., Remigi, P., & Adams, G. C. (2011). Phytophthora species in forest streams in Oregon and Alaska. Mycologia, 103, 22–35. https://doi.org/10.3852/10-013
Riddell, C. E., Frederickson-Matika, D., Armstrong, A. C., Elliot, M., Forster, J., Hedley, P. E., Morris, J., Thorpe, P., Cooke, D. E. L., Pritchard, L., Sharp, P. M., & Green, S. (2019). Metabarcoding reveals a high diversity of woody host-associated Phytophthora spp. in soils at public gardens and amenity woodlands in Britain. PeerJ, 7, e6931. https://doi.org/10.7717/peerj.6931
Riolo, M., Aloi, F., La Spada, F., Sciandrello, S., Moricca, S., Santilli, E., Pane, A., & Cacciola, S. O. (2020). Diversity of Phytophthora communities across different types of Mediterranean vegetation in a nature reserve area. Forests, 11, 853. https://doi.org/10.3390/f11080853
Robin, C., Piou, D., Feau, N., Douzon, G., Schenck, N., & Hansen, E. M. (2011). Root and aerial infections of Chamaecyparis lawsoniana by Phytophthora lateralis: A new threat for European countries. Forest Pathology, 41, 417–424. https://doi.org/10.1111/j.1439-0329.2010.00688.x
Rodríguez-Padrón, C., Rodríguez, A., & Siverio, F. (2019). Survey in Nurseries and Irrigation Water Reservoirs as Sources of Oomycetes Found in Avocado Orchards in the Canary Islands. Plant Disease, 103, 1264–1274. https://doi.org/10.1094/PDIS-08-18-1412-RE
Rossmann, S., Lysøe, E., Skogen, M., Talgø, V., & Brurberg, M. B. (2021). DNA metabarcoding reveals broad presence of plant pathogenic oomycetes in soil from internationally traded plants. Frontiers in Microbiology, 12, 637068. https://doi.org/10.3389/fmicb.2021.637068
Ruiz Gómez, F. J., Navarro-Cerrillo, R. M., Pérez-de-Luque, A., Oβwald, W., Vannini, A., & Morales-Rodríguez, C. (2019). Assessment of functional and structural changes of soil fungal and oomycete communities in holm oak declined dehesas through metabarcoding analysis. Scientific Reports, 9, 5315. https://doi.org/10.1038/s41598-019-41804-y
Runia, W. T., Michielsen, J. M. G. P., Van Kuik, A. J., & Van Os, E. (1997). Elimination of root-infecting pathogens in recirculating water by slow sand filtration. In Proceedings of the 9th international congress on soilless cultures, Jersey 12-19 April 1997 (pp. 395–407).
Rytkonen, A., Lilja, A., Petaisto, R.-L., & Hantula, J. (2008). Irrigation water and Phytophthora cactorum in a forest nursery. Scandinavian Journal of Forest Research, 8(23), 404–411. https://doi.org/10.1080/02827580802419034
Safaiefarahani, B., Mostowfizadeh-Ghalamfarsa, R., Hardy, G. E. S. J., & Burgess, T. I. (2015). Re-evaluation of the Phytophthora cryptogea species complex and the description of a new species, Phytophthora pseudocryptogea sp. nov. Mycological Progress, 14, 108. https://doi.org/10.1007/s11557-015-1129-9
Santilli, E., Riolo, M., La Spada, F., Pane, A., & Cacciola, S. O. (2020). First report of root rot caused by Phytophthora bilorbang on Olea europaea in Italy. Plants, 9, 826. https://doi.org/10.3390/plants9070826
Sarker, S. R., Burgess, T. I., Hardy, G. E. S. J., & McComb, J. (2023a). Closing the gap between the number of Phytophthora species isolated through baiting a soil sample and the number revealed through metabarcoding. Mycological Progress, 22, 39. https://doi.org/10.1007/s11557-023-01892-7
Sarker, S. R., McComb, J., Hardy, G. E. S. J., & Burgess, T. I. (2023b). Sample volume affects the number of Phytophthora and Pythium species detected by soil baiting. European Journal of Plant Pathology, 166, 303–313. https://doi.org/10.1007/s10658-023-02661-8
Sarker, S. R., McComb, J., Burgess, T. I., & Hardy, G. E. S. J. (2021). Timing and abundance of sporangia production and zoospore release influences the recovery of different Phytophthora species by baiting. Fungal Biology, 125(6), 477–484. https://doi.org/10.1016/j.funbio.2021.01.009
Scanu, B., Linaldeddu, B. T., Deidda, A., & Jung, T. (2015). Diversity of Phytophthora species from declining mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE, 10(12), e0143234. https://doi.org/10.1371/journal.pone.0143234
Scanu, B., Hunter, G. C., Linaldeddu, B. T., Franceschini, A., Maddau, L., Jung, T., & Denman, S. (2014). A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. Forest Pathology, 44, 1–20. https://doi.org/10.1111/efp.12064
Schmitz, S., Zini, J., & Chandelier, A. (2009). Involvement of Phytophthora species in the decline of beech (Fagus sylvatica) in the southern part of Belgium. In E. M. Goheen & S. J. Frankel (Eds.), Phytophthoras in forests and natural ecosystems: Fourth meeting of the International Union of Forest Research Organizations (IUFRO) working party S07.02.09 (pp. 320–323). USDA Forest Service, Pacific southwest Research Station, Albany, California. General technical report PSW-GTR-221.
Scibetta, S., Schena, L., Chimento, A., Cacciola, S. O., & Cooke, D. E. L. (2012). A molecular method to assess Phytophthora diversity in environmental samples. Journal of Microbiological Methods, 88, 356–368. https://doi.org/10.1016/j.mimet.2011.12.012
Seddaiu, S., Brandano, A., Ruiu, P. A., Sechi, C., & Scanu, B. (2020). An overview of Phytophthora species inhabiting declining Quercus suber stands in Sardinia (Italy). Forests, 11, 971. https://doi.org/10.3390/f11090971
Shrestha, S. K., Zhou, Y., & Lamour, K. (2013). Oomycetes baited from streams in Tennessee 2010–2012. Mycologia, 105, 1516–1523. https://doi.org/10.3852/13-010
Simamora, A. V., Paap, T., Howard, K., Stukely, M. J. C., Hardy, G. E. S. J., & Burgess, T. I. (2018). Phytophthora contamination in a nursery and is potential dispersal into the natural environment. Plant Disease, 102, 132–139. https://doi.org/10.1094/PDIS-05-17-0689-RE
Stepniewska, H. (2005). Phytophthora spp. on beech seedlings in some forest nurseries of South Poland. In Phytophthora spp. in nurseries and forest stands (pp. 47–52). Forest research institute.
Stępniewska, H., & Dłuszyński, J. (2010). Incidence of Phytophthora cambivora in bleeding lesions on beech stems in selected forest stands in South-Eastern Poland. Phytopathologia, 56, 39–51.
Taberlet, P., Coissac, E., Pompanon, F., Brochmann, C., & Willerslev, E. (2012). Towards next-generation biodiversity assessment using DNA metabarcoding. Molecular Ecology, 21, 2045–2050. https://doi.org/10.1111/j.1365-294X.2012.05470.x
Telfer, K. H., Brurberg, M. B., Herrero, M.-L., Stensvand, A., & Talgø, V. (2015). Phytophthora cambivora found on beech in Norway. Forest Pathology, 45, 415–425. https://doi.org/10.1111/efp.12215
Themann, K., Werres, S., Lüttmann, R., & Diener, H. A. (2002). Observations of Phytophthora spp. in water recirculation systems in commercial hardy ornamental nursery stock. European Journal of Plant Pathology, 108, 337–343. https://doi.org/10.1023/A:1015614625414
Tsao, P. H. (1990). Why many Phytophthora root rots and crown rots of tree and horticultural crops remain undetected. EPPO Bulletin, 20, 11–17. https://doi.org/10.1111/j.1365-2338.1990.tb01174.x
Ufer, T., Werres, S., Posner, M., & Wessels, H. - P. (2008). Filtration to eliminate Phytophthora spp. from recirculating water systems in commercial nurseries. Plant Health Progress, 9(1). https://doi.org/10.1094/PHP-2008-0314-01-RS
Vannini, A., Bruni, N., Tomassini, A., Franceschini, S., & Vettraino, A. M. (2013). Pyrosequencing of environmental soil samples reveals biodiversity of the Phytophthora resident community in chestnut forests. FEMS Microbiology Ecology, 85, 433–442. https://doi.org/10.1111/1574-6941.12132
Van Os, E. A., Wohanka, W., Bruins, M., & Seidel, R. (2000). Slow filtration: A technique to minimise the risks of spreading root-infecting pathogens in a closed hydroponic system. Acta Horticularae, 559, 495–502. https://doi.org/10.17660/ActaHortic.2001.559.72
Vettraino, A. M., Barzanti, G. P., Bianco, M. C., Ragazzi, A., Capretti, P., Paoletti, E., Luisi, N., Anselmi, N., & Vannini, A. (2002). Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. Forest Pathology, 32, 19–28. https://doi.org/10.1046/j.1439-0329.2002.00264.x
Vettraino, A. M., Morel, O., Perlerou, C., Robin, C., Diamandis, S., & Vannini, A. (2005). Occurrence and distribution of Phytophthora species associated with ink disease of chestnut in Europe. European Journal of Plant Pathology, 111, 169–180. https://doi.org/10.1007/s10658-004-1882-0
VÚLHM. (2007). Zpravodaj ochrany lesa: Aktuální problémy ochrany lesa, 14. Výzkumný ústav lesního hospodářství a myslivosti.
White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (Vol. 18, pp. 315–322). Academic press, Inc.. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Yang, X., & Hong, Ch. (2013). Phytophthora virginiana sp. nov., a high-temperature tolerant species from irrigation water in Virginia. Mycotaxon, 126, 167–176. https://doi.org/10.5248/126.167
Acknowledgements
The authors are grateful to all nursery owners for their collaboration in collecting soil and water samples and providing information about nurseries. Matěj Pánek (Institute of Microbiology, Czech Academy of Sciences, Prague) and Miloň Dvořák (Mendel University in Brno) are acknowledged for supplying several soil samples for this research. The authors acknowledge the Research/Scientific Computing teams at The James Hutton Institute and NIAB for providing computational resources and technical support for the “UK’s Crop Diversity Bioinformatics HPC” (BBSRC grant BB/S019669/1), use of which has contributed to the results reported within this paper. The authors are grateful to Peter Hedley and Jenny Morris at The James Hutton Institute for sequencing services.
Funding
Open access publishing supported by the National Technical Library in Prague. The work of the team from Mendel University in Brno (MENDELU) was supported by the European Regional Development Fund, Project Phytophthora Research Centre Reg. No. CZ.02.1.01/0.0/0.0/15_003/0000453 and the internal MENDELU project IGA LDF_VP_2019019. Staff at The James Hutton Institute acknowledge funding from The Rural and Environment Science and Analytical Services Division of the Scottish Government.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and samples collection and processing were performed by A. Bačová, I. Milenkovič, T. Májek, Z. Nagy and T. Corcobado. Sample Analysis - A. Bačová, Z. Nagy, E. Randall, B. Keillor, M. Horta Jung, and M. Tomšovský. Data analysis, software, data curation - P. Cock and D. Cooke. Writing - review and editing - M. Tomšovský, D. Cooke, and T. Jung. The first draft of the manuscript was written by A. Bačová, and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bačová, A., Cooke, D.E.L., Milenković, I. et al. Hidden Phytophthora diversity unveiled in tree nurseries of the Czech Republic with traditional and metabarcoding techniques. Eur J Plant Pathol 170, 131–156 (2024). https://doi.org/10.1007/s10658-024-02886-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-024-02886-1