Abstract
This review assesses possible reasons for the discrepancy between the high numbers of Phytophthora species and putative new species isolated from environmental samples using metabarcoding, compared with the low number cultured and identified through bating. Molecular protocols are unlikely to result in high numbers of false positives, except that variants in young hybrid species may be incorrectly identified as different species. Baiting conditions favour parasitic species that are fast to sporulate, able to infect a range of bait species, achieve infection with a low number of zoospores, and fast-growing on selective agar. Species may not be isolated because they are slow-growing saprophytes and slow to sporulate when baited. Changes to protocols that might result in the isolation of more species include changes in the timing of exposure of baits, inclusions of dead baits, reducing potential competition from fast-growing species by baiting only small volumes of soil, and isolation on media without antimicrobials. However, the species not isolated may have growth traits precluding easy isolation, such as host specificity or obligate biotroph lifestyle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1995, there were around 60 described Phytophthora species, and almost all were associated with diseases of food crops (Erwin and Ribeiro 1996). The genus now includes 223 described species, both pathogens and non-pathogens, which occur widely in cultivated and natural ecosystems (Abad et al. 2022). Scott et al. (2019) have suggested that this number may double due to the exploration of natural ecosystems. Recognition of the increased number of species has been due in part to investigations of previously unexplored natural ecosystems but also due to the application of molecular taxonomy, which allows discrimination between different species that exhibit the same or very similar morphology, identification of hybrids and recognition of new species that may not have been previously cultured (Abad et al. 2022).
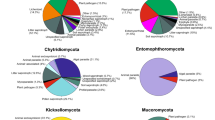
The number of species detected in an environmental sample through metabarcoding of environmental DNA (eDNA) using high throughput sequencing (HTS) platforms is much higher than the number isolated by traditional methods such as baiting in water using floating leaves, seedling roots or fruits (Burgess et al. 2017). The discrepancy is not confined to reports from a particular country, vegetation type, or the molecular tools used, as shown by a summary of eight recent studies (Fig. 1, Table 1, Supp. Table 1). In these studies, on average, only ~ 25% of the total species detected (using both molecular methods and baiting) were isolated, and ~ 20% of the total were uncultured species. Two of the most extensive studies illustrate the differences in the number of species detected using the two methodologies. Khdiar et al. (2020) studied soils from different urban forests in Perth, Western Australia. From 236 samples, baiting techniques detected Phytophthora species in 24 samples, while metabarcoding using the internal transcribed spacer 1 (ITS1) region of rRNA detected Phytophthora in 168 samples. Forty-four Phytophthora phylotypes were detected from these samples, but only four species were isolated. In an extensive investigation of the Phytophthora species in Britain, Landa et al. (2021) detected Phytophthora in 91 locations over 14 sites. They used baiting and HTS, using primers for the ITS1 region of rRNA and the mitochondrial cytochrome c oxidase I (CO1) gene. Sixty-one species were detected; 23 could be identified as species or hybrids, but 38 were unknown in culture and possibly new species. Only 8 species were isolated through baiting, and in only two locations were the data from HTS and baiting in agreement. In the eight studies shown in Supp. Table S1, very few species, except P. multivora, were always isolated if detected using molecular methods, even commonly occurring species such as P. cambivora, P. cinnamomi, P. cryptogea, and P. nicotianae. The discrepancy was seen for species from all phylogenetic clades.
Phytophthora species detected only through metabarcoding and not from baiting include known culturable species and those that have never been isolated. The unculturable species can be of ecological importance. For example, P. taxon ballota is particularly interesting as it is widespread and often the dominant species associated with Spain’s evergreen holm oak decline, yet it has never been isolated (Català et al. 2017). When all species detected from an area of disease cannot be isolated, the contribution of each species to vegetation health and disease cannot be resolved. The problems arising from the non-isolation of species detected by molecular methods also apply to Phytopythium and Nothophytophthora species, which, although mainly non-pathogenic, may include some pathogens (Chen et al. 2022; Landa et al. 2021). For many purposes, isolation by traditional methods is essential as living cultures make possible the study of biology, ecology, pathogenicity, epidemiology, and management of Phytophthora diseases. Metabarcoding using eDNA only confirms the presence of an organism in a particular habitat; it does not indicate if the organism is viable or pathogenic (Català et al. 2015; Burgess et al. 2017). Phytophthora species can vary greatly in physiological attributes, so a species isolated from one location cannot be assumed to be the same physiologically as isolates of the same species from another locality. More understanding of the soil microbial profile and its taxonomic and metabolic fluctuations can be obtained using metatranscriptomics (Segata et al. 2013). However, these methods give a broad-brush picture and cannot replace glasshouse and field experiments using isolates of Phytophthora.
This review discusses two intermingled problems: Why are so many known culturable species not routinely isolated by techniques such as baiting when molecular analysis shows them to be present in a soil sample, and why are there so many unculturable species? Possible reasons for the number of species detected being higher using molecular techniques than the conventional baiting methods include errors or shortcomings in the molecular techniques, the biology and physiology of the different Phytophthora species, and the adequacy of the baiting conditions and isolation media. This review examines possible factors contributing to the success of isolation and protocol changes that may increase recoveries.
Factors associated with the molecular techniques
Molecular techniques may result in false positives or false negatives. An investigation by Riddell et al. (2019) illustrated that both false positives and, less commonly, false negatives might occur from control mixtures. Landa et al. (2021) analyzed a control mixture of 12 Phytophthora species. They only failed to detect one, but the species detected differed depending on whether the ITS1 or COI gene region was used for the assessment. Burgess et al. (2022) tested mock communities containing more species than any previous study. No false positives were observed from any of the primers tested. In general, the false negatives were due to the low DNA concentration of some species in the mock community. False negatives may have been due to insufficient primer annealing or because some closely related species have an identical or very similar sequence and cannot be differentiated if they occur in the same sample (for example Català et al. 2015; Burgess et al. 2017; Bose et al. 2018).
False positives are unlikely to result from contamination, as all studies include a no template PCR control during the amplification phase and laboratory contamination would be detected in this sample. Most recent studies also use an internal control of either known species of Phytophthora or a ‘synthetic’ Phytophthora. These are spiked into samples and allow detection of cross-contamination during the amplification stage (Green et al. 2020, 2021). However, hybrid species may lead to false positives. A recent, young hybrid that is still evolving may display many different related ITS sequences and appear as several species and increase the number of ‘species’ present, but on the other hand, primers may not detect a hybrid if one or both parent species are also present (Burgess 2015; Burgess et al. 2022).
An extensive study of the results from different primer sets used in recently published studies on Phytophthora (Burgess et al. 2022) showed wide variation in their ability to identify Phytophthora species in mock communities and environmental samples. Primers for the ITS1 region, which are widely used, gave the best results. However, although this region reliably separates species from a wide array of organisms, more information on the degree of intraspecific variation in this region in Phytophthora species is needed to reduce the possibility of false positives. Additionally, some closely related species may have an identical ITS1 sequence. Thus, amplifying at least two gene regions would improve confidence in species assignment. Overall false positives are likely to be rare and account for few of the additional species or putatively new species detected using HTS compared to baiting.
Molecular analysis but not baiting may detect DNA from dead hyphae or reproductive structures, as DNA can persist in the soil after the death of an organism (Kunadiya et al. 2021). However, such instances will be rare and usually of little relevance as investigators are interested in the viable microbes in the soil.
Factors associated with the biology and physiology of Phytophthora species
Mode of nutrition of Phytophthora species
Phytophthora species have a wide range of growth modes. Failure to isolate a species may be because it is an obligate biotroph such as P. polygoni (Zheng and Ho 1998) or has a growth mode not yet identified amongst Phytophthora species, such as being a mycoparasite, obligate endophyte, or a species-specific obligate biotroph—all growth modes that are present in Pythium, Peronospora, and other oomycetes (Berry et al. 1993; Fang and Tsao 1995; Cooke et al. 2000; Spring et al. 2018).
Saprophytic ability varies considerably between Phytophthora species, from ones mainly saprophytic (e.g., many Clade 6 species) to species such as P. cinnamomi, which are poor competitive saprophytes (McCarren 2006). Aram and Rizzo (2018) showed that P. gonapodyides, when competing with P. ramorum, could not colonize live rhododendron bait leaves, but it out-competed P. ramorum on dead leaves. Adding dead leaves as well as fresh baits may allow the isolation of additional species, especially saprophytes.
Most pathogenic species of Phytophthora are either necrotrophs or have a variable period of biotrophy before switching to necrotrophy (Cahill et al. 2008). However, species such as P. cinnamomi, P. kernoviae and P. ramorum can grow biotrophically or endophytically in some plant species resulting in asymptomatic infections (Crone et al. 2013a, b; Fichtner et al. 2012). Some species may also asymptomatically infect bait leaves (Huberli et al. 2000; 2008, Sarker et al. 2023). Metabarcoding analysis of asymptomatic bait leaves has detected non-culturable Phytophthora species and those isolated from lesioned leaves in the same tubs (Sarker et al. 2023). As a species’ DNA on an asymptomatic bait must have resulted from the encystment of swimming zoospores, it is unclear why these species do not all grow out from these baits when plated. Some species may be obligate biotrophs, and the selective agar medium is not appropriate, or they are very slow growing, so they get missed. It is also possible that the zoospores have died after encystment as they could not infect the bait leaf, and their residual DNA is being detected.
Host specificity
Host specificity may contribute to the failure of isolation through baiting as some species may require bait leaves or the presence of root exudates of a particular species for successful baiting. Almost half of the soil-borne Phytophthora species are known to cause disease on only one or two host species, almost all of which are dicotyledons (Scott et al. 2019). A low number of recorded hosts may be due to the rarity of the species and the fact that additional hosts have not been tested rather than true species specificity. Also, host specificity in the field may not preclude zoospores from infecting leaves of other species during baiting. However, even if some species can infect only a narrow range of bait species, this may not prevent their zoospores from encysting on contact with the bait and being detected when DNA from asymptomatic baits is analyzed.
Speed of production of zoospores from baited material
The time required for sporangial production and zoospore release after the commencement of baiting and the abundance of zoospores produced by different Phytophthora species is a possible reason for some species being challenging to isolate (Sarker et al. 2021). Phytophthora species were found to vary widely in the timing of sporangia production, release of zoospores and their abundance when infected tissue was submerged and baited. Species that produced sporangia and zoospores quickly from lesions (within 8–12 h) were found to infect and produce lesions on baits early and could be isolated more easily than species that required more time to produce zoospores. Most species that were fast to produce zoospores also produced them in high numbers, further exacerbating the problem of isolating species that were slower to sporulate. Further, when species fast in sporangia and zoospore production, such as P. cinnamomi, P. multivora, P. nicotianae, P. pseudocryptogea, and P. thermophila were combined in baiting tubs with species slower to sporulate, as predicted, the faster sporulating species infected more baits and were thus easier to isolate than the slower species (Sarker et al. 2021).
Methods of overcoming the problem of isolating multiple species from a sample containing species with different times of sporulation include the possibility of daily harvesting of baits and the provision of fresh baits, or in some other way reducing the competition for baits within a tub.
Factors associated with baiting protocols and isolation media
Mixing samples and volume of soil baited
When surveying an area for the presence of Phytophthora species, a commonly used protocol for preparing the baiting sample is to collect small samples of soil and roots from beneath diseased trees and shrubs, then mix all the samples from around each tree or from a site. Subsamples of this mixture are then baited (Burgess et al. 2021; Pérez-Sierra et al. 2022). This protocol maximizes the chances of multiple species of Phytophthora occurring in the same tub, increases interspecific competition and thus increases the likelihood of failing to isolate some species. Sarker et al. (2023) compared the recovery of species from mixed soil samples from a site, and from baiting small individual soil samples. Higher numbers of Phytophthora (and Phytopythium) species were recovered when samples were baited separately rather than from bulked soil. However, the number of species detected still did not reach the number recorded from metabarcoding. Hence, species differences in abundance and timing of sporangial development and zoospore release are not the only factors responsible for the observed differences.
Pretreatment of samples
Slow-growing or dormant propagules may not be isolated, but their DNA will be detected. Various pretreatments of samples, such as drying the soil or exposure to a low temperature, have increased the number of recoveries (Pérez-Sierra et al. 2022). Airdrying and rewetting soil samples before baiting increased the numbers of P. cactorum isolations (Jeffers and Aldwinckle 1987), while Davison and Tay (2005) showed drying the soil after an initial baiting period, then rewetting and rebaiting (double baiting) increased the number of isolates of P. cinnamomi, P. citricola, and P. megasperma. Double baiting small samples and using fresh baits daily may be a sequence worth investigating. For P. ramorum, storage of soil samples at 4 °C or 30 days before baiting significantly increased recoveries (Tooley and Carras 2011), but Osterbauer et al. (2015) did not confirm this. The collection season will influence the type of propagules in the soil and determine whether a vernalization treatment is effective for some species. Data on factors that stimulate the germination of dormant oospores or chlamydospores are highly desirable. However, for many species, the difficulty/impossibility of obtaining large numbers of such spores for experimentation precludes experimental investigation.
Water depth
The rationale underlying the baiting protocol is that flooding separates the material infected with Phytophthora from the floating baits and that while motile Phytophthora zoospores can reach the baits, many other contaminating microbes with non-motile propagules will be unable to do so. However, the water depth must be appropriate for the species of Phytophthora present as different species have different swimming speed patterns and longevity (Appiah et al. 2005). A depth of 2–5 cm and a soil–water ratio of 1:3 is usual for baiting trays when floating leaf baits are applied, But the optima for these variables have not been determined for the different species, and this could be an important factor in the competition between species for baits. Martin et al. (2012) suggests that a lower water-to-soil ratio is advantageous when the inoculum level is low. Jeffers and Aldwinckle (1987) found that small changes in the volume of water significantly affected the proportion of baits infected with P. cactorum. The number of spores needed to result in an infection varies between species, and in some species, zoospores aggregate strongly (Kasteel et al. 2023). The volume of water may impact the ability of zoospores of some species to reach the baits in sufficient numbers before being outcompeted by other species able to infect with lower numbers of spores.
Temperature
Temperature is highly likely to affect Phytophthora species differentially and change their competitive ability. The temperature has a marked effect on sporulation and mycelial growth in vivo and in vitro. Temperature optima for mycelial growth, sporangial production, and zoospore release may differ between isolates of a species and vary between species. The temperature range for mycelial growth is more often known than that for sporangial production and zoospore release, which frequently has a narrower range. For example, Khaliq et al. (2020) found that P. cinnamomi mycelium grows between 7.5 and 35 °C, produces sporangia from 12 to 30 °C and releases zoospores over an even narrower temperature range; 15 to 27.5 °C. Many baiting experiments are conducted at 20–25 °C or uncontrolled ‘room temperature’.
For soil-borne pathogenic species of Phytophthora, their growth rate and the temperature range over which mycelial growth will occur is very wide: For example, P. austrocedrae grows between 2 and 25 °C and has a maximum growth rate of 1.5 mm/day at 17.5 °C (Greslebin et al. 2007) while P. balyanboodja grows between 7.5 and 40 °C and has a growth rate of 14 mm/d at the temperature optimum of 32.5 °C (Burgess et al. 2018). Even though these data are for mycelial growth and not sporangial production or zoospore release, the wide range and the observation that species with maximum growth at a lower temperature exhibit a slow growth rate suggest that using different temperatures to pretreat soil, incubate baiting trays and culture the plated bait leaves, may result in the isolation of additional species. However, it is possible that even at a temperature optimal for a slow-growing species but sub-optimal for a fast-growing one, the latter will still grow significantly faster and out-compete the former. Additional knowledge of the temperature profiles for sporangial production and zoospore release and whether a threshold temperature is required to trigger sporangial production is needed to determine conditions most favourable for isolating challenging species. A range of temperatures for pretreating samples and during baiting may enable the isolation of new species shown from the molecular analysis.
The temperature may also affect whether sporangia of some species release zoospores or germinate directly (Pfender et al. 1977). Although direct germination is most common in Phytophthora species that infect foliage or fruit (Clerk 1972; Kessel et al. 2009), it has been recorded in soil-borne species such as P. megasperma. If baiting temperatures promote direct germination of some species, they will be less competitive for bait infection than species producing zoospores.
Bait preference/manipulation
Further improvements to the number of species isolated may result from using different bait species. Bait species have been extensively investigated over many years but have mainly utilized dicotyledonous angiosperm plant parts, and leaves which give better results than roots or cotyledons (Pérez-Sierra et al. 2022). Leaves of several susceptible angiosperms or a mixture of angiosperm and conifer needles are commonly used depending on the vegetation present. Entire leaves rather than wounded ones are preferable, as wounding stimulates bait infection by Pythium and bacteria (Ghimire et al. 2009), but cut leaf discs are frequently used for species with large leaves or when the experimental design requires the bait area to be equal under different experimental conditions. Of course, the species susceptible to Phytophthora species that have not been isolated are unknown, and these uncultured species may have a narrow host range. As mentioned above, more data would be desirable for the bait species parasitized by Phytophthora species with only one or two host species. Most species pathogenic on gymnosperms are also pathogenic on some angiosperm species. However, two soil-borne species, P. pseudotsuga, and P. abietivora, have only gymnosperm hosts suggesting some species may not be isolated when only angiosperm baits are offered. In diseased areas where conifers, cycads and ferns are present, including the baits of these plants may aid the isolation of any Phytophthora species specific to these phyla. Zoospores respond to chemotactic and electrotactic stimuli, and the same stimulus may repel or attract different species. Understandably most past research has concerned the successful baiting of pathogenic species. Stimuli that attract pathogenic species may repel nonpathogenic ones (Kasteel et al. 2023), adding an extra layer of complexity to selecting appropriate baits.
Composition of isolation media
Compared with competitive fungi and bacteria, the relatively slow growth in vitro of most Phytophthora species has resulted in the use of selective media for plating bait lesions. The constituents of such media have a combination of antimicrobials at concentrations non-toxic, or at least less inhibitory to Phytophthora than to the contaminants. These media were primarily developed, and the concentrations of the antimicrobials were extensively tested when relatively few Phytophthora species were available. It is now known that hymexazol and chloramphenicol inhibit the growth of some Phytophthora species at concentrations used in some isolation media (Hansen et al. 1979; Jeffers and Martin 1986; Tsao and Guy 1977). However, a study of the growth of the 47 species of Phytophthora occurring in Australia in the presence of concentrations of the antimicrobials present in a widely used isolation medium NARPH (Hüberli et al. 2000) found that growth was reduced to the extent that it could be expected to make isolation difficult in only three species; P. cactorum, P. parvispora, and P. sojae (Sarker et al. 2020). Most analyses of sensitivity to antimicrobials have only been conducted using hyphal growth as a measure of sensitivity. However, in environmental samples, the propagules present may be chlamydospores, oospores or other resting structures, and their tolerance levels may be very different from those of mycelium (Mircetich 1970).
Although the use of antibiotics in the isolation medium does not appear to be the main impediment to isolation, improving the Phytophthora selective medium remains desirable. New compounds should be screened to identify those that inhibit contaminating organisms but not Phytophthora. A prospective list of such compounds arises from the study by Lawrence et al. (2017). They screened 120 compounds with antimicrobial activity (including antibiotics, cations, anions, and chelators) to inhibit P. cinnamomi and P. agathidicida, 88 of the compounds screened did not affect the two Phytophthora species. Many of the compounds listed in Supplementary Table 1 of Lawrence et al. (2017) deserve further investigation as possible selective inhibitors of contaminants and may improve isolation media for Phytophthora. Enhanced control of microbial contaminants may allow the use of a more nutrient-rich agar for isolation, thus increasing the growth of the slow-growing Phytophthora species. Of course, sensitivity to antimicrobials can only be tested using species already in culture, and failure to isolate some species may be because they are highly sensitive. Plating baits on media with no antimicrobials and attempting early isolations of small uncontaminated hyphal tips may result in isolating species highly sensitive to one or more antimicrobials or which are quickly overgrown with contaminant organisms or other Phytophthora species. It is possible to isolate Phytophthora using carrot or water agar without antimicrobials, but it takes more time and care than when using selective agar (Erwin and Ribeiro 1996).
Analysis of asymptomatic baits and filtered bait water
DNA analysis of either asymptomatic baits or of the filters from filtering bait water frequently reveals species in addition to those detected through DNA analysis of soil and roots; these include species known in culture and uncultured species. Plating of the asymptomatic baits or the filters has the problem that they will also carry fast-growing competitive Phytophthora species and other contaminants, and plating results in only small additional numbers of species in culture (Khaliq et al. 2018). The filters may pick up species that do not adhere to the available baits, but filters will only have the zoospores present on the day of filtering. It is possible that filtering damages or kills fragile zoospores of some species. Analysis of asymptomatic baits is more straightforward than filters and may reveal species in the water over the days the baits were exposed.
The role of multiple Phytophthora species in the ecosystem
Until proven otherwise, we must assume that the ‘rules’ of ecology and evolution are the same for above- and below-ground organisms. Thus, no two organisms with identical ecological requirements can coexist in the same niche, and some form of reproductive isolation is required for divergence and speciation. The unexpectedly high number of Phytophthora species from the same habitat and the occurrence of up to four Phytophthora species in a diseased plant (at least in nursery container plants) (Hardy and Sivasithamparam 1988) raises the questions of how all these species exist sympatrically.
-
1.
What role does each play in the ecosystem?
-
2.
What is their host range, and does it include herbaceous annuals as well as woody perennials?
-
3.
Which are saprophytes and which are pathogens, and what is their level of virulence?
-
4.
Do the benign species compete with the pathogens either in the soil or in planta, and if so, could we manipulate the environment to favour them rather than the pathogenic ones?
-
5.
Is there any form of chemical inhibition between the multiple Phytophthora species in the rhizosphere?
-
6.
How significant are the species that we cannot culture?
Molecular tools might be used to pinpoint the locations in the ecosystem occupied by different species, including uncultured ones, and indicate if they occur in plant tissues either as endophytes or in association with disease and how frequently two (or more) pathogenic species are present together in a root. However, a complete understanding of the ecology of Phytophthora species in the soil will require cultures for experimental analysis.
Conclusions
The disparity between the number of Phytophthora species identified from an environmental sample using molecular techniques and the number that can be isolated using baiting impedes a full understanding of the subterranean ecosystem and constrains the development of management protocols. Species present but not isolated include species known in culture and putative new species, and explanations for the differences in numbers must cover both these species groups. Investigations are restricted by the obvious fact that we can only experiment using species already in the culture. It does seem possible that the uncultured species may exhibit a broader range of growth traits than present in the known species, perhaps more equivalent to those seen in Pythium and Peronospora. Experiments using laboratory mixtures of Phytophthora species have shown that errors and false positives during the metabarcoding analysis appear unlikely, and molecular protocols are more likely to lead to false negatives than false positives. Refinement of primers, assessment of more than one genetic region, and complete knowledge of the intraspecific genetic variation of the regions assessed will result in some of the currently putative new species being shown to be variants of existing species. Features of reproductive biology and physiology that impinge on the likelihood of a species being baited successfully include the speed of sporangial production and zoospore release, the stimuli required to induce germination of resting spores, and the species’ growth responses to different temperatures and water depths. The suitability of baits or the presence of antimicrobials in the isolation medium do not appear to be major factors for the species already in culture, but these factors could be important for the unculturable, putative new species. More information is needed regarding the ability of the species with only one or two recorded host species to infect leaves of commonly used bait species. Such species comprise a large proportion of the cultured soil-borne Phytophthora species.
It is hypothesized that changes to baiting protocols that result in less competition between species will allow the isolation of more species (at least more of those known in culture). Such changes could include collecting small individual samples of soil and baiting small volumes of soil, frequently harvesting and replacing baits, inclusion of dead baits and baiting at more than one temperature. Some uncultured species might be recovered if new antimicrobial compounds are developed for selective media or a medium with no antimicrobials is used. Only when we can reliably isolate most species in an ecosystem can we expect to understand the interactions between the plethora of Phytophthora species in subterranean ecosystems and their impacts on disease expression.
Data availability
Data availability and sharing not applicable to this article as all data are from cited publications.
References
Abad ZG, Burgess TI, Redford AJ, Bienapfl JC, Srivastava S, Mathew R, Jennings K (2022) IDphy: an International online resource for molecular and morphological identification of Phytophthora based on type specimens. Plant Dis. https://doi.org/10.1094/PDIS-1002-1022-0448-FE
Appiah AA, Van West P, Osborne MC, Gow NAR (2005) Potassium homeostasis influences the locomotion and encystment of zoospores of plant pathogenic oomycetes. Fungal Genet Biol 42(3):213–223. https://doi.org/10.1016/j.fgb.2004.11.003
Aram K, Rizzo DM (2018) Distinct trophic specializations affect how Phytophthora ramorum and clade 6 Phytophthora spp. colonize and persist on Umbellularia californica leaves in streams. Phytopathology 108(7):858–869. https://doi.org/10.1094/PHYTO-06-17-0196-R
Berry LA, Jones EE, Deacon JW (1993) Interaction of the mycoparasite Pythium oligandrum with other Pythium species. Biocontrol Sci Techn 3(3):247–260. https://doi.org/10.1080/09583159309355280
Bose T, Wingfield MJ, Roux J, Vivas M, Burgess TI (2018) Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol 36:17–25. https://doi.org/10.1016/j.funeco.2018.09.001
Burgess TI (2015) Molecular characterization of natural hybrids formed between five related indigenous clade 6 Phytophthora species. PLoS ONE 10(8):e0134225. https://doi.org/10.1371/journal.pone.0134225
Burgess TI, White D, McDougall KL, Garnas J, Dunstan WA, Català S, Carnegie AJ, Worboys S, Cahill D, Vettraino A-M, Stukely MJC, Liew ECY, Paap T, Bose T, Migliorini D, Williams B, Brigg F, Crane C, Rudman T, Hardy GES (2017) Distribution and diversity of Phytophthora across Australia. Pac Conserv Biol 23:150–162. https://doi.org/10.1071/PC16032
Burgess TI, Simamora A, White D, Williams B, Schwager M, Stukely MJC, Hardy GESJ (2018) New species from Phytophthora Clade 6a: evidence for recent radiation. Persoonia - Mol Phylogeny Evol Fungi 41:1–17. https://doi.org/10.3767/persoonia.2018.41.01
Burgess TI, López-Villamor A, Paap T, Williams B, Belhaj R, Crone M, Dunstan WA, Howard K, Hardy GES (2021) Towards a best practice methodology for the detection of Phytophthora species in soils. Plant Pathol 70:604–614. https://doi.org/10.1371/journal.pone.0134225
Burgess TI, White D, Sapsford SJ (2022) Comparison of primers for the detection of Phytophthora (and other oomycetes) from environmental samples. J Fungi 8:980. https://doi.org/10.3390/jof8090980
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Turner Review No. 17. Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Aust J Bot 56:279–310. https://doi.org/10.1071/BT07159
Català S, Pérez-Sierra A, Abad-Campos P (2015) The use of genus-specific amplicon pyrosequencing to assess Phytophthora species diversity using eDNA from soil and water in northern Spain. PLoS ONE 10:e0119311. https://doi.org/10.1371/journal.pone.0119311
Català S, Berbegal M, Pérez-Sierra A, Abad-Campos P (2017) Metabarcoding and development of new real-time specific assays reveal Phytophthora species diversity in holm oak forests in eastern Spain. Plant Pathol 66:115–123. https://doi.org/10.1111/ppa.12541
Chen Q, Bakhshi M, Balci Y, Broders KD, Cheewangkoon R, Chen SF, Fan XL, Gramaje D, Halleen F, Horta Jung M, Jiang N, Jung T, Májek T, Marincowitz S, Milenković I, Mostert L, Nakashima C, Nurul Faziha I, Pan M, Raza M, Scanu B, Spies CFJ, Suhaizan L, Suzuki H, Tian CM, Tomšovský M, Úrbez-Torres JR, Wang W, Wingfield BD, Wingfield MJ, Yang Q, Yang X, Zare R, Zhao P, Groenewald JZ, Cai L, Crous PW (2022) Genera of phytopathogenic fungi: GOPHY 4. Stud Mycol 101:417–564. https://doi.org/10.3114/sim.2022.101.06
Clerk GC (1972) Germination of Sporangia of Phytophthora palmivora (Butl.) Butl. Ann Bot 36:801–807. https://doi.org/10.1093/oxfordjournals.aob.a084636
Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM (2000) A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet Biol 30(1):17–32. https://doi.org/10.1006/fgbi.2000.1202
Crone M, McComb JA, O’Brien PA, Hardy GES (2013a) Annual and herbaceous perennial native Australian plant species are symptomless hosts of Phytophthora cinnamomi in the Eucalyptus marginata (jarrah) forest of Western Australia. Plant Path 62:1057–1062. https://doi.org/10.1111/ppa.12016
Crone M, McComb JA, O’Brien PA, Hardy GES (2013b) Assessment of Australian native annual/herbaceous perennial plant species as asymptomatic or symptomatic hosts of Phytophthora cinnamomi under controlled conditions. Forest Pathol 43:245–251. https://doi.org/10.1111/efp.12027
Davison EM, Tay FCS (2005) How many soil samples are needed to show that Phytophthora is absent from sites in the south-west of Western Australia? Australas Plant Pathol 34:293–297
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. APS Press, St. Paul, Minnesota
Fang JG, Tsao PH (1995) Evaluation of Pythium nunn as a potential biocontrol agent against Phytophthora root rots of azalea and sweet orange. Phytopathology 85:29–36
Fichtner EJ, Rizzo DM, Kirk SA, Webber JF (2012) Infectivity and sporulation potential of Phytophthora kernoviae to select North American native plants. Plant Pathol 61:224–233. https://doi.org/10.1111/j.1365-3059.2011.02506.x
Ghimire SR, Richardson PA, Moorman GW, Lea-Cox JD, Ross DS, Hong CX (2009) An in-situ baiting bioassay for detecting Phytophthora species in irrigation runoff containment basins. Plant Pathol 58:577–583. https://doi.org/10.1111/j.1365-3059.2008.02016.x
Green S, Riddell CE, Frederickson-Matika D, Armstrong A, Elliot M, Forster J, Hedley PE, Morris J, Thorpe P, Cooke DE, Sharp P, Pritchard L (2020) Diversity of woody-host infecting Phytophthora species in public parks and botanic gardens as revealed by metabarcoding, and opportunities for mitigation through best practice. Sibbaldia 18:67–88
Green S, Cooke DEL, Dunn M, Barwell L, Purse BV, Chapman DS, Valatin G, Schlenzig A, Barbrook J, Pettitt T, Price C, Pérez-Sierra A, Frederickson-Matika D, Pritchard L, Thorpe P, Cock PJA, Randall E, Keillor B, Marzano M (2021) PHYTO-THREATS: addressing threats to UK forests and woodlands from Phytophthora; identifying risks of spread in trade and methods for mitigation. Forests 12(12):1617. https://doi.org/10.3390/f12121617
Greslebin AG, Hansen EM, Sutton W (2007) Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentina). Mycol Res 111:308–316. https://doi.org/10.1016/j.mycres.2007.01.008
Hansen EM, Hamm PB, Julis AJ, Roth LF (1979) Isolation, incidence and management of Phytophthora in forest tree nurseries in the Pacific Northwest. Plant Dis Rep 63(7):607–611
Hardy GES, Sivasithamparam K (1988) Phytophthora spp. associated with container-grown plants in nurseries in Western Australia. Plant Dis 72:435–437
Hüberli D, Tommerup IC, Hardy GES (2000) False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australasian Plant Pathol 29:164–169. https://doi.org/10.1071/AP00029
Hüberli D, Lutzy B, Voss B, Calver M, Ormsby M, Garbelotto M (2008) Susceptibility of New Zealand flora to Phytophthora ramorum and pathogen sporulation potential: an approach based on the precautionary principle. Australas Plant Path 37:615–625. https://doi.org/10.1071/APO8064
Jeffers SN, Aldwinckle HS (1987) Enhancing detection of Phytophthora catorum in naturally infested soil. Phytopathology 77(10):1475–1482
Jeffers SN, Martin SB (1986) Comparison of two media selective for Phytophthora and Pythium species. Plant Dis 70:1038–1043. https://doi.org/10.1094/PD-70-1038
Kasteel M, Ketelaar T, Govers F (2023) Fatal attraction: How Phytophthora zoospores find their host. Seminars in Cell and Developmental Biology: Academic Press. https://doi.org/10.1016/j.semcdb.2023.01.014
Kessel GJT, Veloso S, Forch MG, Latorse M-P (2009) New Phytophthora populations: a shift from indirect to direct sporangial germination? Proceedings of the Eleventh EuroBlight Workshop, Hamar, Norway, 28-31 October 2008, pp 171-176
Khaliq I, Hardy GESJ, White D, Burgess TI (2018) eDNA from roots: a robust tool for determining Phytophthora communities in natural ecosystems FEMS. Microbial Ecol 94:fiy048. https://doi.org/10.1093/femsec/fiy048
Khaliq I, Hardy GESJ, Burgess TI (2020) Phytophthora cinnamomi exhibits phenotypic plasticity in response to cold temperatures. Mycol Prog 19:405–415. https://doi.org/10.1007/s11557-020-01578-4
Khdiar MY, Barber PA, Hardy GESJ, Shaw C, Steel EJ, McMains C, Burgess TI (2020) Association of Phytophthora with declining vegetation in an urban forest environment. Microorganisms 8:973. https://doi.org/10.3390/microorganisms8070973
Kunadiya M, Burgess TI, Dunstan WA, White D, Hardy GESJ (2021) Persistence and degradation of Phytophthora cinnamomi DNA and RNA in different soil types. Environ DNA 3:92–104. https://doi.org/10.1002/edn3.127
La Spada F, Cock PJA, Randall E, Pane A, Cooke DEL, Cacciola SO (2022) DNA metabarcoding and isolation by baiting complement each other in revealing Phytophthora diversity in anthropized and natural ecosystems. J Fungi 8(4):330. https://doi.org/10.3390/jof8040330
Landa BB, Arias-Giraldo LF, Henricot B, Montes-Borrego M, Shuttleworth LA, Pérez-Sierra A (2021) Diversity of Phytophthora species detected in disturbed and undisturbed british soils using high-throughput sequencing targeting ITS rRNA and COI mtDNA r egions. Forests 12:229. https://doi.org/10.3390/f12020229
Lawrence SA, Armstrong CB, Patrick WM, Gerth ML (2017) High-throughput chemical screening identifies compounds that inhibit different stages of the Phytophthora agathidicida and Phytophthora cinnamomi life cycles. Front Microbiol 8:1340. https://doi.org/10.3389/fmicb.2017.01340
Martin FN, Abad ZG, Balci Y, Ivors K (2012) Identification and detection of Phytophthora: reviewing our progress, identifying our needs. Plant Dis 96:1080–1103. https://doi.org/10.1094/PDIS-12-11-1036-FE
McCarren KL (2006) Saprophytic ability and the contribution of chlamydospores and oospores to the survival of Phytophthora cinnamomi. PhD dissertation Murdoch University Western Australia
Mircetich SM (1970) Inhibition of germination of chlamydospores of Phytophthora cinnamomi by some antimicrobial agents in Phytophthora selective media. Can J Microbiol 16(12):1227–1230. https://doi.org/10.1139/m70-206
Osterbauer NK, Navarro S, Lane S, Trippe A (2015) Assessing the effect of vernalization on the detection of Phytophthora ramorum from native soil, potting media, and cull piles in Oregon nurseries. Plant Health Prog 16(1):50–51. https://doi.org/10.1094/PHP-BR-14-0038
Pérez-Sierra A, Horta Jung M, Jung T (2022) Survey and monitoring of Phytophthora species in natural ecosystems: methods for sampling, isolation, purification, storage, and pathogenicity tests. In: Luchi N (ed) Plant Pathology: Method and Protocols. Springer, New York, pp 13–49. https://doi.org/10.1007/978-1-0716-2517
Pfender WF, Hine RB, Stanghellini ME (1977) Production of sporangia and release of zoospores by Phytophthora megasperma in soil. Phytopathology 67:657–663
Riddell CE, Frederickson-Matika D, Armstrong AC, Elliot M, Forster J, Hedley PE, Morris J, Thorpe P, Cooke DEL, Pritchard L, Sharp PM, Green S (2019) Metabarcoding reveals a high diversity of woody host-associated Phytophthora spp. in soils at public gardens and amenity woodlands in Britain. PeerJ 7:e6931. https://doi.org/10.7717/peerj.6931
Sarker SR, McComb JA, Burgess TI, Hardy GES (2020) Antimicrobials in Phytophthora isolation media and the growth of Phytophthora species. Plant Pathol 69:1426–1436. https://doi.org/10.1111/ppa.13224
Sarker SR, McComb J, Burgess TI, Hardy GES (2021) Timing and abundance of sporangia production and zoospore release influences the recovery of different Phytophthora species by baiting. Fungal Biol 125:477–484. https://doi.org/10.1016/j.funbio.2021.01.009
Sarker SR, McComb J, Hardy GESJ, Burgess TI (2023) Sample volume affects the number of Phytophthora and Phytopythium species detected by soil baiting. Eur J Plant Pathol. https://doi.org/10.1007/s10658-023-02661-8
Scott P, Bader M, Burgess TI, Hardy GESJ, Williams N (2019) Global biogeography and invasion risk of the plant destroyer genus Phytophthora. Environ Sci Policy 101:175–182. https://doi.org/10.1016/j.envsci.2019.08.020
Segata N, Boernigen D, Tickle TL, Morgan XC, Garrett WS, Huttenhower C (2013) Computational meta’omics for microbial community studies. Mol Syst Biol 9(1):666. https://doi.org/10.1038/msb.2013.22
Spring O, Gomez-Zeledon J, Hadziabdic D, Trigiano RN, Thines M, Lebeda A (2018) Biological characteristics and assessment of virulence diversity in pathosystems of economically important biotrophic oomycetes. Crit Rev Plant Sci 37(6):439–495. https://doi.org/10.1080/07352689.2018.1530848
Tooley PW, Carras MM (2011) Enhanced recovery of Phytophthora ramorum from soil following 30 days of storage at 4 C. Journal of Phytopathl 159(9):641–643. https://doi.org/10.1111/j.1439-0434.2011.01810.x
Tsao PH, Guy SO (1977) Inhibition of Mortierella and Pythium in a Phytophthora-isolation medium containing hymexazol. Phytopathology 67:796–801
Vannini A, Bruni N, Tomassini A, Franceschini S, Vettraino AM (2013) Pyrosequencing of environmental soil samples reveals biodiversity of the Phytophthora resident community in chestnut forests. FEMS Microbiol Ecol 85:433–442. https://doi.org/10.1111/1574-6941.12132
Zheng X, Ho HH (1998) The rediscovery of Phytophthora polygoni Saw. Botanical Bulletin of Academia Sinica 39:209–212
Acknowledgements
SR Sarker gratefully acknowledges receipt of a Research Training Programme Scholarship from Murdoch University, Australia
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Suchana Rani Sarker was supported by a Murdoch University Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
SR Sarker prepared the initial manuscript which was then developed further by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Tanay Bose
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarker, S.R., Burgess, T.I., Hardy, G.E.S.J. et al. Closing the gap between the number of Phytophthora species isolated through baiting a soil sample and the number revealed through metabarcoding. Mycol Progress 22, 39 (2023). https://doi.org/10.1007/s11557-023-01892-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01892-7