Abstract
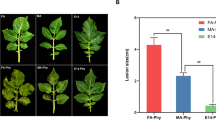
The Ny-1 gene confers hypersensitive response (HR) to Potato virus Y (PVY) in potato cultivars (Solanum tuberosum L.). Tetraploid potato breeding clone PB07–37 possessing the allele Ny-1 in a duplex state was developed. After PVY infection, the size of necrotic lesions in leaves of PB07–37 was reduced by approximately 68% in relation to cultivar Rywal plants displaying Ny-1 in a simplex dosage. Two-dimensional gel electrophoresis (2-DE) was applied for screening PVY-induced proteins in leaves of PB07–37. Compared with non-infected control plants, 60 reproducible PVY-induced proteins were detected using LC-MS/MS analysis, of which 41 were involved in qualitative changes and 19 were differently expressed in inoculated leaves by at least 1.5-fold. Proteins involved in the category of photosynthesis and primary metabolism were the most abundant. The results from PB07–37 (Ny-1 duplex) were compared with data from Rywal (Ny-1 simplex). The protein profiles in the Ny-1 simplex and Ny-1 duplex plants are genotype specific. Only eight proteins were identified in both genotypes. Five of them: ATP synthase CF1 alpha chain, chloroplastic; ATP synthase CF1 beta subunit, chloroplastic; ATP synthase subunit beta, mitochondrial-like; linoleate 13S-lipoxygenase 2–1, chloroplastic; and mitochondrial monodehydroascorbate reductase 5 are known to be involved in the defense response in plants. In our study, however, protein abundance did not correspond to the Ny-1 resistance gene dosage.



Similar content being viewed by others
References
Alexander, M. M., & Cilia, M. (2016). A molecular tug-of-war: Global plant proteome changes during viral infection. Current Plant Biology, 5, 13–24.
Bengtsson, T., Weighill, D., Proux-Wéra, E., Levander, F., Resjö, S., Burra, D. D., Moushib, L. I., Hedley, P. E., Liljeroth, E., Jacobson, D., Alexandersson, E., & Andreasson, E. (2014). Proteomics and transcriptomics of the BABA-induced resistance response in potato using a novel functional annotation approach. BMC Genomics, 15, 315.
Blée, E. (2002). Impact of phyto-oxylipins in plant defense. Trends in Plant Science, 7, 315–321.
Chai, Q., Shang, X., Wu, S., Zhu, G., Cheng, C., Cai, C., Wang, X., & Guo, W. (2017). 5-Aminolevulinic acid dehydratase gene dosage affects programmed cell death and immunity. Plant Physiology, 175, 511–528.
Chivasa, S., Murphy, A. M., Hamilton, J. M., Lindsey, K., Carr, J. P., & Slabas, A. R. (2009). Extracellular ATP is a regulator of pathogen defence in plants. The Plant Journal, 60, 436–448.
Collmer, C. W., Marston, M. F., Taylor, J. C., & Jahn, M. (2000). The I gene of bean: A dosage-dependent allele conferring extreme resistance, hypersensitive resistance, or spreading vascular necrosis in response to the potyvirus Bean common mosaic virus. Molecular Plant-Microbe Interactions, 13, 1266–1270.
Croft, K. P. C., Voisey, C. R., & Slusarenko, A. J. (1990). Mechanism of hypersensitive cell collapse: Correlation of increased lipoxygenase activity with membrane damage in leaves of Phaseolus vulgaris (L.) inoculated with an avirulant race of Pseudomonas syringae pv. phaseolicola. Physiological and Molecular Plant Pathology, 36, 49–62.
Flis, B., Hennig, J., Strzelczyk-Żyta, D., Gebhardt, C., & Marczewski, W. (2005). The Ry-f sto gene from Solanum stoloniferum for extreme resistant to Potato virus Y maps to potato chromosome XII and is diagnosed by PCR marker GP122718 in PVY resistant potato cultivars. Molecular Breeding, 15, 95–101.
Gebhardt, C., & Valkonen, J. P. T. (2001). Organization of genes controlling disease resistance in the potato genome. Annual Review of Phytopathology, 39, 79–102.
Hackett, C. A., Bradshaw, J. E., Meyer, R. C., McNicol, J. W., Milbourne, D., & Waugh, R. (1998). Linkage analysis in tetraploid species: A simulation study. Genetics Research, 71, 143–154.
Hatsugai, N., Koldenkova, V. P., Imamura, H., Noji, H., & Nagai, T. (2012). Changes in cytosolic ATP levels and intracellular morphology during bacteria-induced hypersensitive cell death as revealed by real-time fluorescence microscopy imaging. Plant and Cell Physiology, 53, 1768–1775.
Henkes, S., Sonnewald, U., Badur, R., Flachmann, R., & Stitt, M. (2001). A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell, 13, 535–551.
Jia, L., Bai, J., Guan, D., Sun, K., Jiao, Q., & Feng, H. (2016). Extracellular ATP: A potential molecule regulating the defence response of plants to biotic stresses – A review. Plant Protection Science, 52, 221–228.
Kangasjärvi, S., Neukermans, J., Li, S., Aro, E.-M., & Noctor, G. (2012). Photosynthesis, photorespiration, and light signalling in defence responses. Journal of Experimental Botany, 63, 1619–1636.
Li, D., Chen, P., Shi, A., Shakiba, E., Gergerich, R., & Chen, Y. (2009). Temperature affects expression of symptoms induced by soybean mosaic virus in homozygous and heterozygous plants. Journal of Heredity, 100, 348–354.
Raffaele, S., Leger, A., & Roby, D. (2009). Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant Signaling &. Behavior, 4, 94–99.
Ramakers, C., Ruijter, J. M., Lekanne Deprez, R. H., & Moorman, A. F. M. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters, 339, 62–66.
Rance, I., Fournier, J., & Esquerré-Tugayé, M.-T. (1998). The incompatible interaction between Phytophthora parasitica var. nicotianae race 0 and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proceedings of the National Academy of Sciences of USA, 95, 6554–6559.
Ribeiro, A. M., Pinto, C. A. B. P., Andrade, C. M., dos Santos, J. B., & Figueira, A. R. A. (2006). SCAR marker for the selection of Ry-duplex potato clones immune to potato virus Y. Crop Breeding and Applied Biotechnology, 6, 1–8.
Rühle, T., & Leister, D. (2015). Assembly of F1F0-ATP synthases Biochimica et Biophysica. Acta, 1847, 849–860.
Sagredo, B. D., Mathias, M. R., Barrientos, C. P., Acuña, I. B., Kalazich, J. B., & Santos, J. R. (2009). Evaluation of a SCAR RYSC3 marker of the Ry adg gene to select resistant genotypes to Potato virus Y (PVY) in the INIA potato breeding program. Chilean Journal of Agricultural Research, 69, 305–315.
Scholthof, K. B., Adkins, S., Czosnek, H., Palukaitis, P., Jacquot, E., Hohn, T., Hohn, B., Saunders, K., Candresse, T., Ahlquist, P., Hemenway, C., & Foster, G. D. (2011). Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology, 12, 938–954.
Scranton, M. A., Yee, A., Park, S.-Y., & Walling, L. L. (2012). Plant leucine aminopeptidases moonlight as molecular chaperones to alleviate stress-induced damage. The Journal of Biological Chemistry, 287, 18408–18417.
Solomon-Blackburn, R. M., & Bradshaw, J. E. (2007). Resistance to Potato virus Y in a multitrait potato breeding scheme without direct selection in each generation. Potato Research, 50, 87–95.
Szajko, K., Chrzanowska, M., Witek, K., Strzelczyk-Żyta, D., Zagórska, H., Gebhardt, C., Hennig, J., & Marczewski, W. (2008). The novel gene Ny-1 on potato chromosome IX confers hypersensitive resistance to Potato virus Y and is an alternative to Ry genes in potato breeding for PVY resistance. Theoretical and Applied Genetics, 116, 297–303.
Szajko, K., Strzelczyk-Żyta, D., & Marczewski, W. (2018). Comparison of leaf proteomes of potato (Solanum tuberosum L.) genotypes with ER- and HR-mediated resistance to PVY infection. European Journal of Plant Pathology, 150, 375–385.
Szarzynska, B., Sobkowiak, L., Pant, B. D., Balazadeh, S., Scheible, W. R., Mueller-Roeber, B., Jarmolowski, A., & Szweykowska-Kulinska, Z. (2009). Gene structures and processing of Arabidopsis thaliana HYL1-dependent primiRNAs. Nucleic Acids Research, 37, 3083–3093.
Umate, P. (2011). Genome-wide analysis of lipoxygenase gene family in Arabidopsis and rice. Plant Signaling &. Behavior, 6, 335–338.
Valkonen, J. P. T. (2015). Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato. Breeding Science, 65, 69–76.
Vogel, H., & Marcotte, E. M. (2012). Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Review Genetics, 13, 227–232.
Zhao, J., Zhang, X., Hong, Y., & Liu, Y. (2016). Chloroplast in plant-virus interaction. Frontiers in Microbiology, 7, 1565.
Zheng, C., Chen, P., & Gergerich, R. (2005). Effect of temperature on the expression of necrosis in soybean infected with Soybean mosaic virus. Crop Science, 45, 916–922.
Acknowledgements
The research was supported by The National Science Centre in Poland, grant UMO-2014/13/B/NZ9/02468.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Human and/or animals rights
Not applied.
Informed consent
Not applied.
Rights and permissions
About this article
Cite this article
Szajko, K., Sołtys-Kalina, D., Szarzynska, B. et al. A comparative proteomic analysis of the PVY-induced hypersensitive response in leaves of potato (Solanum tuberosum L.) plants that differ in Ny-1 gene dosage. Eur J Plant Pathol 153, 385–396 (2019). https://doi.org/10.1007/s10658-018-1565-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1565-x