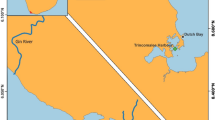
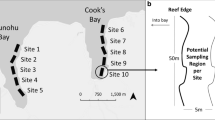
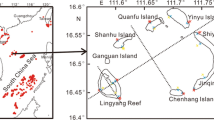
Abstract
Species-specific spatial distribution in relation to environmental characteristics underpins the species diversity of coral reef fishes. This study aimed to elucidate (1) the broad-scale spatial distribution (spatial variation of fish density at intervals of several-kilometers), influenced by topographic features (exposed reef vs. inner reef), substrate characteristics and depth, and (2) the microhabitat associations (habitat association within several centimeter scale) concerning substrate availability for seven angelfish species (family Pomacanthidae) in an Okinawan coral reef. Broad-scale analysis revealed (1) Chaetodontoplus mesoleucus was primarily found in deep inner reefs with greater coverage of branching Acropora and dead coral; (2) Centropyge bicolor and C. tibicen were primarily found at shallow inner reefs with greater coverage of branching Acropora, dead coral, and sand; (3) C. ferrugata and C. vrolikii were primarily found at shallow exposed reefs with greater coverage of rock, and (4) C. heraldi and Pygoplites diacanthus were primarily found at deep exposed reefs with greater coverage of rock. Microhabitat-scale analysis revealed that three species (C. mesoleucus, C. bicolor, and C. heraldi) showed significant positive association with acroporid corals. Centropyge tibicen showed a significant positive association with living corals. The remaining three species (C. ferrugata, C. vrolikii, and P. diacanthus) did not show a positive association with living corals. This suggests that coral loss impacts angelfish population in a species-specific manner. These two spatial scale viewpoints offer valuable insight for comprehensive understandings of angelfish spatial distribution in relation to substrate characteristics.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aldenhoven JM (1986) Different reproductive strategies in a sex-changing coral reef fish Centropyge bicolor (Pomacanthidae). Ayst J Mar Freshw Res 37:353–360
Alwany MA (2009) Distribution and feeding ecology of the angelfishes (Pomacanthidae) in Shalateen region, Red Sea, Egypt. Egypt J Aquat Biol Fish 13:79–91
Alwany MA (2012) Diversity of butterfly and angel fishes assemblages around Zabargad Island, Red Sea, Egypt. Egypt J Exp Biol 8:181–189
Benthuysen JA, Emslie MJ, Currey-Randall LM, Cheal AJ, Heupel MR (2022) Oceanographic influences on reef fish assemblages along the Great Barrier Reef. Prog Oceanogr 208:102901
Bouchon-Navaro Y (1986) Partitioning of food and space resources by chaetodontid fishes on coral reefs. J Exp Mar Biol Ecol 103:21–40
Cheal A, Emslie M, Miller I, Sweatman H (2012) The distribution of herbivorous fishes on the Great Barrier Reef. Mar Biol 159:1143–1154
Doll PC, Munday PL, Bonin MC, Jones GP (2021) Habitat specialization and overlap in coral reef gobies of the genus Eviota (Teleostei: Gobiidae). Mar Ecol Prog Ser 677:81–94
Donaldson TJ (2002) Habitat association and depth distribution of two sympatric groupers of the genus Cephalopholis (Serranidae: Epinephelinae). Ichthyol Res 49:191–193
Eagle JV, Jones GP, McCormick MI (2001) A multi-scale study of the relationship between habitat use and the distribution and abundance patterns of three coral reef angelfishes (Pomacanthidae). Mar Ecol Prog Ser 214:253–265
Emslie MJ, Pratchett MS, Cheal AJ, Osborne K (2010) Great Barrier Reef butterflyfish community structure: the role of shelf position and benthic community type. Coral Reefs 29:705–715
Eurich J, McCormick MI, Jones GP (2018) Habitat selection and aggression as determinants of fine-scale partitioning of coral reef zones in a guild of territorial damselfishes. Mar Ecol Prog Ser 587:201–215
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Roders KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305
Froese R, Pauly D (2022) FishBase. https://www.fishbase.org. Accessed 27 Oct 2022
Fulton CJ, Bellwood DR, Wainwright PC (2001) The relationship between swimming ability and habitat use in wrasses (Labridae). Mar Biol 139:25–33
Green AL (1996) Spatial, temporal and ontogenetic patterns of habitat use by coral reef fishes (Family Labridae). Mar Ecol Prog Ser 133:1–11
Green AL, Maypa AP, Almany GR, Rhodes KL, Weeks R, Abesamis RA, Gleason MG, Mumby PJ, White AT (2015) Larval dispersal and movement patterns of coral reef fishes, and implication for marine reserve network design. Biol Rev 90:1215–1247
Green A, White A, Kilarski S (2013) Designing marine protected area networks to achieve fisheries, biodiversity, and climate change objectives in tropical ecosystems: a practitioner guide. The nature Conservancy, and the USAID Coral Triangle Support Pertnership, Cebu City, Phillippines. viii+35pp
Gust N, Choat JH, McCormick MI (2001) Spatial variability in reef fish distribution, abundance, size and biomass: a multi-scale analysis. Mar Ecol Prog Ser 214:237–251
Hernández-Landa RC, Acosta-González G, Núñez-Lara E, Arias-González JE (2014) Spatial distribution of surgeonfish and parrotfish in the north sector of the Mesoamerican Barrier Reef System. Mar Ecol 36:452–446
Hoey AS, Bellwood DR (2008) Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 27:37–47
Hourigan TF, Stanton FG, Motta P, Kelley CD, Carlson B (1989) The feeding ecology of three species of Caribbean angelfishes (family Pomacanthidae). Environ Biol Fish 24:105–116
Johnson GB, Taylor BM, Robbins WD, Franklin EC, Toonen R, Bowen B, Choat JH (2019) Diversity and structure of parrotfish assemblages across the northern Great Barrier Reef. Diversity 11:14
Kelleher G (1999) Guidelines for Marine protected Areas. IUCN, Gland, p xxiv+107
Khalaf MA, Abdallah M (2014) Spatial distribution of fifty ornamental fish species on coral reefs in the Red Sea and Gulf of Aden. ZooKeys 367:33–64
Lange ID, Benkwitt CE, McDevitt-Irwin JM, Tietjen KL, Taylor B, Chinkin M, Gunn RL, Palmisciano M, Steyaert M, Wilson B, East HK, Turner J, Graham NAJ, Perry CT (2021) Wave exposure shapes reef community composition and recovery trajectories at a remote coral atoll. Coral Reefs 40:1819–1829
Lindquist DG, Gilligan MR (1986) Distribution and relative abundance of angelfishes and angelfishes across a lagoon and barrier reef, Andros Island, Bahamas. Northeast Gulf Sci 8:1–8
Loya Y, Sakai K, Yamazato K, Nakano H, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Luckhurst BE, Luckhurst K (1978) Analysis of the influence of substrate variables on coral reef fish communities. Mar Ecol Prog Ser 49:317–323
Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP (2002) Resource selection by animals: statistical design and analysis for field studies, 2nd edn. Kluwer, Dordrecht
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: Differential susceptibility among taxa. Coral Reefs 19:155–163
McClanahan TR, Baird AH, Marshall PA, Toscano MA (2004) Comparing bleaching and mortality responses of hard corals between southern Kenya in the Great Barrier Reef, Australia. Mar Poll Bull 48:327–335
McCormick MI (1994) Comparison of field methods for measuring surface topography and their associations with a tropical reef fish assemblage. Mar Ecol Prog Ser 112:87–96
Moyer JT (1990) Social and reproductive behavior of Chaetodontoplus mesoleucus (Pomacanthidae) at Bantayan island, Philippines, with notes on pomacanthid relationships. Japan J Ichthyol 36:459–467
Munday PL, Jones GP, Caley MJ (1997) Habitat specialization and the distribution and abundance of coral-dwelling gobies. Mar Ecol Prog Ser 152:227–239
Nanami A (2018) Spatial distributions, feeding ecologies, and behavioral interactions of four rabbitfish species (Siganus umimaculatus, S. virgatus, S. corallinus, and S. piellus). PeerJ 6:e6145
Nanami A (2020) Spatial distribution and feeding substrate of butterflyfishes (family Chaetodontidae) on an Okinawan coral reef. PeerJ 8:e9666
Nanami A (2021) Spatial distribution of parrotfishes and groupers in an Okinawan coral reef: size-related associations in relation to habitat characteristics. PeerJ 9:e12134
Nanami A, Nishihira M, Suzuki T, Yokochi H (2005) Species-specific habitat Distribution of coral reef fish assemblages in relation to habitat characteristics in an Okinawan coral reef. Environ Biol Fish 72:55–65
Nanami A, Sato T, Takebe T, Teruya K, Soyano K (2013) Microhabitat association in white-streaked grouper Epinephelus ongus: importance of Acropora spp. Mar Biol 160:1511–1517
Newman SJ, Williams DMcB, Russ G (1997) Patterns of zonation of assemblages of the Lutjanidae, Lethrinidae and Serranidae (Epinephelinae) within and among mid-shelf and outer-shelf reefs in the central Great Barrier Reef. Mar Freshwater Res 48:119–128
Okemwa GM, Fulanda B, Ochiewo J, Kimani EN (2004) Exploitation of coral reef fishes for the marine aquarium trade in Kenya: a preliminary assessment. Final Tech. Rep 19/2004, pp 42. http://aquadocs.org/bitstream/handle/1834/8990/ktf00ex02.pdf?sequence=1&isAllowed=y. Accessed 27 Oct 2022
Pratchett MS, Berumen ML (2008) Interspecific variation in distributions and diets of coral reef butterflyfishes (Teleostei: Chaetodontidae). J Fish Biol 73:1730–1747
Pratchett MS, Munday PL, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR (2008) Effects of climate-induced coral bleaching on coral-reef fishes –ecological and economic consequences. Oceanogr Mar Biol Annu Rev 46:251–296
Pratchett MS, Schenk TJ, Baine M, Syms C, Baird AH (2009) Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay, Papua New Guinea. Mar Environ Res 67:230–236
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 25 July 2023
Roberts CM, Shepherd ARD, Ormond RFG (1992) Broad-scale variation in assemblage structure of Red Sea angelfishes and angelfishes. J Biogeography 19:239–250
Russ G (1984) Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. II. Patterns of zonation of mid-shelf and outershelf reefs. Mar Ecol Prog Ser 20:35–44
Sakai Y, Kohda M (1997) Harem structure of the protogynous angelfish, Centropyge ferrugatus (Pomacanthidae). Environ Biol Fish 49:333–339
Sato M, Nanami A, Bayne CJ, Makino M, Hori M (2020) Changes in the potential stocks of coral reef ecosystem services following coral bleaching in Sekisei lagoon, southern Japan: implications for the future under global warming. Sustain Sci 15:863–883
Syms C (1995) Multi-scale analysis of habitat association in a guild of blennioids fishes. Mar Ecol Prog Ser 125:31–43
TerBraak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca
Ticzon VS, Mumby PJ, Samaniego BR, Bejarano-Chavarro S, David LT (2012) Microhabitat use of juvenile coral reef fish in Palau. Environ Biol Fish 95:355–370
Tissot BN, Hallacher LE (2003) Effects of aquarium collectors on coral reef fishes in Kona, Hawaii. Conserv Biol 17:1759–1768
Vitelli F, Hyndes GA, Saunders BJ, Blake D, Newman SJ, Hobbs JPA (2019) Do ecological traits of low abundance and niche overlap promote hybridization among coral-reef angelfishes? Coral Reefs 38:931–943
Williams DM (1991) Patterns and processes in the distribution of coral reef fishes. In: Sale PF (ed) The ecology of the fishes on coral reefs. Academic Press, San Diego, pp 437–474
Williams GJ, Smith JE, Conklin EJ, Gove JM, Sala E, Sandin SA (2013) Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1:e81
Wilson SK, Burgess SC, Cheal A, Emslie M, Fisher R, Miller I, Polunin NVC, Sweatman HPA (2008) Habitat use by coral reef fish: implications for specialists vs generalists in a changing environment. J Anim Ecol 77:220–228
Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary R, Tinker P (2010) Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: the importance of coral and algae. PLoS ONE 5:e15185
Wood EM (2001) Collection of coral reef fish for aquaria: global trade, conservation issues and management strategies Marine Conservation Society, UK, pp 80
Acknowledgements
The author express grateful thanks to Masato Sunagawa, Masamitsu Sunagawa, and Masayuki Uezato for their field guide and piloting the research boat YAEYAMA, Teppei Sagawa, Nobuo Motomiya, Kenta Oishi, Fumihiko Nakamura and Minoru Yoshida for field assistance; and the staff of Yaeyama Field Station of Fisheries Technology Institute for support during the present study. Constructive comments from three anonymous reviewers are much appreciated. I would like to thank Editage (www.editage.jp) for English language editing. The present study complies with the current laws in Japan.
Funding
This study was supported by the Environment Research and Technology Development Fund of the Ministry of the Environment, Japan [grant numbers S-15–3(4): JPMEERF16S11513]; the Grant-in-Aid for Scientific Research (A) from Japan Society for the Promotion of Science [grant number 15H02268].
Author information
Authors and Affiliations
Contributions
Atsushi Nanami conceptualized and designed the study and conducted all formal analysis, data curation and writing.
Corresponding author
Ethics declarations
Ethical approval
No animal testing or no animal sampling was performed during the study.
Field studies
All data was obtained only by field observations, which do not require a field permit in Okinawa.
Competing interests
The author declare that have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest
The author declare no completing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Fig. S1
Total number of individuals for each species during the survey period (June 2016- February 2017). Red dashed line represent threshold between dominant and non-dominant species. (PNG 2901 kb)

Fig. S2
Workflow diagram of the present study. (PNG 4673 kb)

Fig. S3
Relative frequency (%) of fish individuals associated with substrates (hard living coral or other substrates) and substrate availability. Numbers above bars represent the number of individuals on the focal substrate. Hard living coral included branching Acropora, corymbose Acropora, tabular Acropora, bottlebrush Acropora, branching coral, foliose coral, massive coral, Pocillopora and other coral. Other substrates included dead coral, soft coral, rock, coral rubble, sand and macroalgae. (PNG 6295 kb)

Fig. S4
Resource selection ratio (wi ± 95% confidence interval) for seven angelfish species. Black and white arrows represent significant positive and non-positive association for the substrates. The vertical dashed line represents a selection ratio of 1 (i.e., no positive or non-positive association). Numbers above bars represent the number of individuals. Substrates with wi ± 95% confidence interval above and below 1 indicate significant positive non-positive association, respectively. Substrates with wi ± 95% confidence interval that encompasses 1 have no significant positive or non-positive association. Hard living coral included branching Acropora, corymbose Acropora, tabular Acropora, bottlebrush Acropora, branching coral, foliose coral, massive coral, Pocillopora and other coral. Other substrates included dead coral, soft coral, rock, coral rubble, sand and macroalgae. (PNG 7360 kb)
ESM 1
(XLS 15 kb)
ESM 2
(XLS 25 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nanami, A. Broad-scale spatial distribution and microhabitat-scale substrate association of seven angelfish species (family Pomacanthidae) in an Okinawan coral reef. Environ Biol Fish 106, 1851–1863 (2023). https://doi.org/10.1007/s10641-023-01461-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01461-7