Abstract
The promising antitumor effects of progesterone derivatives have been identified in many studies. However, the specific mechanism of action of this class of compounds has not been fully described. Therefore, in this study, we investigated the antiproliferative and (anti)estrogenic activities of novel pentacyclic derivatives and benzylidenes of the progesterone series. The antiproliferative effects of the compounds were evaluated on hormone-dependent MCF7 breast cancer cells using the MTT test. Estrogen receptor α (ERα) activity was assessed by a luciferase-based reporter assay. Immunoblotting was used to evaluate the expression of signaling proteins. All benzylidenes demonstrated inhibitory effects with IC50 values below 10 µM, whereas pentacyclic derivatives were less active. These patterns may be associated with the lability of the geometry of benzylidene molecules, which contributes to an increase in the affinity of interaction with the receptor. The selected compounds showed significant anti-estrogenic potency. Benzylidene 1d ((8 S,9 S,10R,13 S,14 S,17 S)-17-[(2E)-3-(4-fluorophenyl)prop-2-enoyl]-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15-decahydrocyclopenta[a]phenanthren-3-one) was the most active in antiproliferative and anti-estrogenic assays. Apoptosis induced by compound 1d was accompanied by decreases in CDK4, ERα, and Cyclin D1 expression. Compounds 1d and 3d were characterized by high inhibitory potency against resistant breast cancer cells. Apoptosis induced by the leader compounds was confirmed by PARP cleavage and flow cytometry analysis. Compound 3d caused cell arrest in the G2/M phase. Further analysis of novel derivatives of the progesterone series is of great importance for medicinal chemistry, drug design, and oncology.
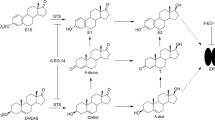
Graphical Abstract

Similar content being viewed by others
Introduction
Steroids are widely distributed in nature, and they have various effects on plant and animal cells [1]. Studies conducted in the 20th century showed that steroids and their metabolism play an extremely important role in human health. Steroidogenesis in humans is a multi-stage process that results in the formation of necessary steroidal products and their delivery to sensitive tissues. Disruption of steroid hormone signaling and activities of steroidogenic enzymes lead to the development of several serious pathologies. Steroids are used to treat many different diseases, including allergic rhinitis, asthma, chronic obstructive pulmonary disease [2, 3], hives and eczema [4], painful joints or muscles (such as arthritis, tennis elbow and frozen shoulder) [5], pain caused by an irritated or trapped nerve (such as sciatica) [6], inflammatory bowel disease (such as Crohn’s disease) [7], systemic lupus erythematosus [8], and multiple sclerosis [9]. The onset of the COVID-19 pandemic in 2020 also highlighted the importance of steroids for human health and medical applications [10, 11]. Dexamethasone was declared a “major development” in the fight against coronavirus disease [12, 13]. Dexamethasone has been described as a recent advancement that significantly reduces the mortality rate among severe COVID-19 patients [12].
Steroids and steroid-like compounds are of particular importance to the progression and treatment of cancers [14]. The transformation of a normal cell into a malignant one is a very complex process. In some cases, steroids play an important supporting role in the growth of malignant cells, including breast and prostate cancer. It has been shown that 60–70% of breast cancers are hormone-dependent, which means that their growth is supported by estrogens. Estrogens, which are steroid hormones and regulate many processes in normal tissues, are converted into “traitors” during the progression of female cancers. When estrogens reach the tumor tissue, rapid cell proliferation occurs. These features of breast cancer have become a focus for many researchers since the 1940s [15, 16]. The discovery of tamoxifen, an effective anti-estrogen, has significantly improved the treatment of breast cancers [17, 18]. Tamoxifen (2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine) is not a steroid molecule, but it has a potent anti-estrogenic effect on hormone-dependent cells [19]. Further development of the series of synthetic steroids produced several effective steroidal anti-estrogens, including fulvestrant [20, 21]. Tamoxifen, fulvestrant, and other anti-estrogens penetrate breast cancer cells and bind to estrogen receptor α (ERα), blocking its interaction with estrogens. “Turning off” estrogen receptor α leads to a decrease in the proliferation rate of breast cancer cells [22, 23].
It was later found that blocking estrogen receptor α is not always sufficient to inhibit the growth of hormone-dependent cancer. In particular, it is very important to reduce the synthesis of estrogens. Thus, inhibitors of aromatase, an enzyme converting androgens to estrogens, have been synthesized [24]. Steroidal and nonsteroidal aromatase inhibitors showing high efficacy in experimental and clinical studies were obtained [25, 26]. Aromatase inhibitor testolactone, which was used as an anticancer drug to treat advanced-stage breast cancer, was discontinued in 2008 [27, 28]. Novel steroidal aromatase inhibitors, including exemestane and formestane, which have been developed on a steroid framework, are currently widely used to treat breast cancer [29].
The antitumor effects of progesterone derivatives have been identified in many studies. Cyproterone acetate is a progesterone derivative with antiandrogenic and progesterone-like activity that has been used in the treatment of advanced prostate cancer [30, 31]. It has not been approved by the Food and Drug Administration for use in the United States but has been approved in other countries. Various targets are considered for progesterone derivatives. Muafia Jabeen and colleagues conducted a pharmacological evaluation and docking studies of progesterone derivatives as anticancer agents [32]. They showed that progesterone derivatives exhibiting antiproliferative activity can bind to estrogen receptor α. In a previous study [33], progesterone derivatives were studied as inhibitors of the 5α reductase enzyme. A phase II study with the progesterone receptor antagonist lonaprisan (ZK 230,211) was performed on progesterone-positive breast cancers. That study examined the efficacy, safety, and tolerability of lonaprisan at two different doses (25 and 100 mg) before the start of phase III trials [34, 35]. The following clinical trials with lonaprisan were discontinued, and further development of the drug was terminated. The progesterone receptor antagonist onapristone has been used as first-line endocrine therapy in breast cancer patients; clinical trials of this drug have been restricted due to its liver toxicity [36]. Dosage forms of onapristone with extended release have been developed. New clinical trials are planned to study letrozole, palbociclib, and extended-release onapristone in patients with metastatic breast cancer (ClinicalTrials.gov ID: NCT04872608). In some cases, approved steroid drugs show adverse effects, low effectiveness, or are characterized by low bioavailability. For example, the clinical efficacy of the lone clinically approved estrogen receptor α degrader, fulvestrant, is limited by its poor oral bioavailability [20]. Although many steroid compounds with anticancer activity have been synthesized, the relevance of new developments in the steroid framework remains high. In this study, we obtained benzylidenes and pentacyclic derivatives of the progesterone series and evaluated their antiproliferative activities and potencies as anti-estrogens in hormone-dependent breast cancer cells.
Materials and methods
Chemistry
We studied the anticancer activities of three classes of steroids derived based on 16-dehydropregnenolone (Table 1). The desired compounds (benzylidenes 1a–h [37] and D-annulated pentacyclic steroids 2a–h [38] and 3b, с, e, and f [39]) were synthesized by previously described methods. General information on these compounds is provided in Sect. 1 of the SI. Synthesis structural characterization including the 1H NMR spectra of novel pentacyclic steroids 3a, d, g, and h are described in Sect. 1.2 of the SI.
Biology
Evaluation of antiproliferative activity
The MCF7 human breast cancer cell line was purchased from the ATCC collection. MCF7 cells were cultured in standard DMEM medium (Gibco) supplemented with 10% fetal bovine serum (FBS, HyClone) at 37 °C, 5% CO2 and 80–85% humidity (NuAire CO2 incubator). The growth of MCF7 cells was evaluated by the modified MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (Applichem) test [40] as described earlier [41]. The resistant cell lines were cultivated according to the method previously described in our work [42]. The cells were seeded at a density of 40 × 103 cells per well in 24-well plates (TPP) in 900 µL of medium. The obtained compounds were dissolved in DMSO (AppliChem) to 5 mM before experiments, and then, the resulting solutions were diluted in the medium to the required concentrations. The viability of the cells after 72-h treatments was assessed after subtraction of the blank value (the absorbance in the well w/o cells) from all wells. Dose–response curves were analyzed by regression analysis using sigmoidal curves (Log(concentration) vs. normalized absorbance). The half-maximal inhibitory concentrations (IC50) were determined with GraphPad Prism.
Luciferase activity
Transfection was performed as described earlier [43] with some modifications. Briefly, MCF7 cells were seeded at a density of 170 × 103 cells per well onto 24-well plates a day before transfection. The next day, the medium was changed from DMEM containing 10% fetal bovine serum (FBS) to phenol red-free DMEM supplemented with 2% dextran-coated charcoal-treated (DCC) serum (steroid-free conditions). Then, the cells were transfected with plasmid containing the luciferase gene controlled by estrogen response elements (ERE). To normalize the transfection efficacy, the β-galactosidase plasmid was co-transfected to the cells along with ERE-Luc. The plasmids used in this study were kindly provided by Frank Gannon and colleagues [44]. Transfection was performed for 4 h with Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s manual. Then, fresh 10% DCC serum-containing phenol red-free DMEM was added to the plates; the studied compounds, which were diluted in medium, were added to the wells as well as the vehicle control and tamoxifen at a concentration of 5 µM. To induce ERα activity, 10 nM 17β-estradiol (Sigma-Aldrich) was used, and it was added 40 min after treatments with the compounds. Cells were lysed within 18 h, and luciferase activity was measured according to the Promega protocol using a Tecan Infinite M200 Pro. The activity of β-galactosidase was analyzed by colorimetric assay using a MultiScan plate reader. The luciferase/β-galactosidase activities were normalized by the internal control values and represented as the mean ± SD value. The relative luciferase activity in 17β-estradiol-treated cells was taken as 100 units. ERα activities were calculated in relative units as the ratio of the luciferase/galactosidase activity.
Immunoblotting
MCF7 breast cancer cells were seeded on 100-mm dishes (Corning), and after 24 h of growth, the compounds were added to a fresh medium. To prepare the cell extracts, MCF7 cells were washed twice in phosphate buffer and incubated for 10 min on ice in modified lysis buffer containing 50 mM Tris-HCl at pH 7.5, 0.5% Igepal CA-630, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.1 mM sodium orthovanadate, aprotinin, leupeptin, and pepstatin (1 µg/mL each) as described earlier [45]. The protein content was determined using the Bradford method [46].
Cell lysates (40 µg of protein) were separated in 10% SDS-PAGE under reducing conditions, transferred to a nitrocellulose membrane (GE HealthCare), and processed according to a standard protocol. To prevent nonspecific absorption, the membranes were treated with 5% nonfat milk solution in TBS buffer (20 mM Tris and 500 mM NaCl at pH 7.5) with 0.1% Tween-20 and then incubated with primary antibodies overnight at 4 °C.
ERα, Cyclin D1, CDK2, CDK4, and PARP antibodies were obtained from Cell Signaling Technology; the antibodies against α-tubulin (Cell Signaling Technology) were added to standardize loading. Goat anti-rabbit IgGs (Jackson ImmunoResearch) conjugated to horseradish peroxidase were used as secondary antibodies. Signals were detected using ECL reagent (as described in Mruk and Cheng’s protocol [47]) and an ImageQuant LAS4000 system (GE HealthCare).
Cell cycle analysis and evaluation of apoptosis
Cell cycle distribution and apoptosis were analyzed as described previously [48]. MCF7 cells were seeded in 6-well plates in DMEM containing 10% FBS and treated with compounds 3d, 2d, and 1d for 24 h. Сell sediments were lysed in a buffer containing 50 µg/mL propidium iodide (Sigma-Aldrich), 100 µg/mL RNase A (Qiagen), 0.1% sodium citrate, and 0.3% NP-40 (Helicon) for 30 min in the dark at 4 °C. Cell cycle and apoptosis data were acquired by measuring DNA content using a Cytoflex flow cytometer 26 (Beckman Coulter) in the PerCP-A channel. At least 20,000 fluorescent ‘events’ were collected for each sample. Data analysis was performed using CytExpert Software (Beckman Coulter).
Statistical evaluation
MS Excel and GraphPad Prism were used to analyze the data. Every experiment was performed at least three individual times to ensure reproducibility, calculate average values and obtain the SD values. ANOVA tests were estimated between groups.
Results and discussion
The antiproliferative activities of the obtained compounds were tested on MCF7 hormone-dependent breast cancer cells. The growth of MCF7 cells is supported by estrogens; thus, anti-estrogens, including some steroids, show high activity against this cancer [49, 50]. To assess the activity of the compounds, the MTT test was performed after 72 h of cell growth with the compounds. The IC50 values are shown in Table 1.
The IC50 values for pentacyclic steroids 3a–h ranged from 7 to 25 µM. The compound with the methoxyphenyl substituent showed weak activity against MCF7 cells. The introduction of halogens into the side chain enhanced the antiproliferative effects of the obtained compounds. The highest activity was shown by a compound containing a fluorophenyl substituent. The activities of chloro-substituted pentacyclic steroids 2a–h were similar. The highest activities were detected for compounds containing 4-chloro- and 4-fluorophenyl substituents at 6 and 7.1 µM, respectively. Interestingly, the introduction of a phenyl group with two halogen substituents led to a decrease in antiproliferative activity (compound 2f). The series of benzylidene derivatives 1a–h was highly active, as shown in Table 1. All benzylidenes demonstrated inhibitory effects with IC50 values below 10 µM. The least active was benzylidene-bearing methoxyphenyl substituent 1 g with an IC50 value of 6.7 µM. As in the case of pentacyclic steroids, benzylidene 1d with the fluorophenyl substituent showed the greatest antiproliferative potency. These patterns may be associated with the geometry of benzylidene molecules. In contrast to pentacyclic steroids, the aryl residue in benzylidenes is not rigidly bound to the steroid residue but has a labile structure, which increases the affinity of the interaction with the receptor due to the lability of the molecular geometry.
Chloro-substituted compounds 3d, 2d, and 1d from the common set of pentacyclic and benzylidene steroids, which showed high activity in the antiproliferative assay, were selected for in-depth analysis. Estrogens in the cell bind to estrogen receptor α (ESR1) [51]. Activation of ERα leads to changes in the expression of many genes, including proliferation regulators. Anticancer compounds may have activating or inhibitory effects on estrogen receptor α. Their activating abilities can be attributed to adverse effects. Using a reporter assay, we tested whether compounds 3d, 2d, and 1d can activate the estrogen receptor in hormone-dependent MCF7 breast cancer cells. A steroid-free medium was used for this assay to minimize the effects of steroids and steroid-like compounds on the transcriptional activity of ERα. As shown in Fig. 1, pentacyclic steroid 3d did not increase the activity of estrogen receptor α, while the natural ligand of the estrogen receptor 17β-estradiol (E2) caused a significant increase in ERα activity. Chloro-substituted pentacyclic steroid 2d and benzylidene 1d showed no agonistic activity. Additionally, the agonistic activity of the anti-estrogen tamoxifen, which in some conditions can significantly activate estrogen receptor α, was not observed [52].
The inhibitory potencies of the compounds were evaluated after activation of the estrogen receptor with 17β-estradiol (Fig. 2). 17β-estradiol caused a significant increase in luciferase activity, which indicates a high activity of estrogen receptor α in MCF7 cells. Compounds 3d, 2d, and 1d at 1 µM did not affect the E2-induced activity of estrogen receptor α. Then, by increasing the concentration of compounds to 5 µM, their anti-estrogenic properties were revealed. Compound 2d at 5 µM suppressed the activity of estrogen receptor α by 43 relative units. Compound 3d was more active, i.e., it blocked estrogen receptor α activity by 71 rel. units. Among the selected compounds, benzylidene 1d showed the greatest activity at a concentration of 5 µM, and estrogen receptor α retained only 14 units of activity. All three selected compounds at a concentration of 15 µM significantly inhibited the activity of estrogen receptor α. The highest activity was observed for benzylidene 1d.
ERα antagonist activity in MCF7 cells. Luciferase reporter assay were performed after 18-h treatments with the compounds at different concentrations (5 µM tamoxifen was used as a reference drug; luciferase was induced by treatments with 10 nM E2). E2–17β-estradiol; *p < 0.05 versus the control sample; #p < 0.05 versus MCF7 cells treated with 10 nM E2 alone
Identification of signaling pathways was performed by immunoblotting. MCF7 cells were treated with the compounds for indicated periods, and then, protein expression was determined by immunoblotting (Fig. 3 A, B).
First, short-term 6-h incubation of MCF7 cells with the compounds was analyzed. In these experiments, no significant changes in protein expression were shown (Fig. 3 A). With an increase in incubation duration to 24 h, compounds 3d and 1d caused a remarkable decrease in ERα expression at a concentration of 15 µM. Treatment with anti-estrogen fulvestrant also caused ERα degradation in MCF7 cells. Compound 1d demonstrated the strongest ERα decrease. Compound 2d triggered a slight decrease in ERα expression under the same conditions. Tamoxifen treatment resulted in a partial increase in ERα expression, which may be attributed to the stabilization of inactivated cytoplasmic ERα [53, 54]. Moreover, all three compounds led to the cleavage of PARP (116 kDa) and the formation of an 89-kDA PARP fragment, which is a key marker of apoptotic cell death [55,56,57]. The effect grew with the increase of the compound concentration from 5 to 15 µM. Then, 89-kDa PARP was significantly increased in MCF7 cells treated with compound 1d at a concentration of 15 µM. The anti-estrogens tamoxifen and fulvestrant did not cause apoptosis in MCF7 cells. Cyclin D1 was analyzed to be an important ERα-dependent gene and cell cycle regulator (Fig. 3B) [58, 59]. A decrease in ERα expression and PARP cleavage in MCF7 cells were accompanied by a slight decrease in cyclin D1 expression at a concentration of 15 µM when fulvestrant treatment did not affect cyclin D1 expression. CDK2 and CDK4 are key regulators of the cell cycle. As shown in Fig. 3B, selected compounds down-regulated CDK4 expression, while CDK2 expression was unchanged.
The cell cycle of the MCF7 cells treated with the leader compounds was analyzed using flow cytometry after staining the cells with a DNA-binding dye propidium iodide (PI). MCF7 cells were incubated with compounds 3d, 2d, and 1d for 24 h, and afterwards, their distribution in different phases of the cell cycle was assessed; MCF7 cells incubated with DMSO were assayed as a control sample (Fig. 4). Apoptotic cells were defined as a sub-G1 peak in the histograms [60, 61].
As shown in Fig. 4, the fraction of cells in the sub-G1 phase of the cell cycle increases in proportion to the increase in the concentrations of compounds 3d, 2d, and 1d. Thus, the antiproliferative activity of the tested compounds is associated precisely with the death of tumor cells of the MCF7 cell line as detected by the cell fraction in the sub-G1 phase. These data correlate well with the apoptosis identified above (Fig. 3B). Interestingly, compound 3d (15 µM) caused cell arrest in the G2/M phase. Several effective chemotherapy drugs and drug candidates cause cell arrest in this phase. These effects have been detected for the following agents: doxorubicin [62], curcumin [63], ribociclib in combination with cisplatin [64], cinobufagin [65], timosaponin AIII [66], apigenin [67], and others.
One of the key problems in oncology is resistance. A course of chemotherapy is usually prescribed after surgery. Many patients are initially sensitive to standard chemotherapy, but with time, cancer cells may develop resistance to the antiproliferative effect of the drugs. In such cases, it is impossible to continue treatment with the chosen drug because the tumor does not respond to this therapy. At this stage, the chemotherapist has to find other drugs that can inhibit tumor growth. Importantly, some drugs can contribute to the development of multidrug resistance. We were interested in whether the resistant cells would be sensitive to the obtained compounds. Cells resistant to several drugs were chosen for the experiments. Cell lines were obtained by prolonged cultivation with the appropriate drug. MCF7/HT, MCF7/DCT, MCF7/CP, and MCF7/RAP cells were characterized by resistance to hydroxytamoxifen, docetaxel, cisplatin, and rapamycin, respectively. Data on the antiproliferative activity of the compounds are shown in Table 2. Compound 1d was less active against resistant lines than against the MCF7 line. Nevertheless, the IC50 value did not exceed 10 µM, indicating a sufficiently high activity of this steroid. Similar data were obtained for compound 2d. The IC50 values for the resistant lines ranged from 11.1 to 17.8 µM. The most interesting data were obtained for compound 3d. This compound was active against MCF7/HT and the parent breast cancer cells (MCF7). The MCF7/DCT and MCF7/RAP breast cancer cells showed higher sensitivity to compound 3d. The activity of compound 3d toward MCF7/CP cells was slightly lower. Thus, the selected compounds are characterized by high activity against resistant cells, exhibiting IC50 values below 10 µM (1d, 3d) and selectivity (3d).
Conclusion
A series of benzylidenes and pentacyclic derivatives of the progesterone series was obtained, and the antiproliferative effects of the compounds on hormone-dependent breast cancer cells were evaluated. The synthesized benzylidenes demonstrated inhibitory effects with IC50 values below 10 µM, and the activities of the benzylidenes were higher than the activities of their pentacyclic analogues. The activities of benzylidene derivatives compared to pentacyclic steroids are likely associated with the lability of the geometry of their molecules, leading to an increase in the affinity of interaction with the receptor.
Estrogen receptor α was studied as a potential target for the selected compounds. No agonistic activity of the compounds on estrogen receptor α was detected in MCF7 cells. The studied compounds showed significant anti-estrogenic properties, with benzylidene 1d being the most active. In addition to potent anti-estrogenic effects, compounds 3d, 2d, and 1d induced apoptosis in MCF7 cells as confirmed by flow cytometry analysis and PARP cleavage. Compound 3d caused cell arrest in the G2/M phase. Steroids 1d and 3d exhibited inhibitory potency toward breast cancer cells with acquired resistance to various anticancer drugs, which makes them important for targeted drug design. Further study of the obtained compounds is of great interest to medicinal chemistry and cancer research.
Data availability
Synthesis and structural characterization of pentacyclic steroids are described in Appendix A. Any other information may be requested directly from the authors (Alex.Scherbakov@gmail.com).
References
Shahbazi Y, Malekinejad H, Tajik H (2016) Determination of naturally occurring estrogenic hormones in cow’s and river buffalo’s meat by HPLC-FLD method. J food drug Anal 24:457–463
Buddala PK, Chandrasekaran V, Harichandrakumar KT (2021) A 3-day course of 1 mg/kg versus 2 mg/kg bodyweight prednisolone for 1- to 5-year-old children with acute moderate exacerbation of asthma: a randomized double-blind noninferiority trial. Paediatrics & child health 26:e189–e193
Ramos-Ramírez P, Tliba O (2022) Glucocorticoid Insensitivity in Asthma: The Unique Role for Airway Smooth Muscle Cells. International journal of molecular sciences 23 (16). DОI:10.3390/ijms23168966
Jing M, Yu Q, Zhu B, Yuan F, Zhang J, Peng L, Lin W, Chen M (2021) Topical 0.05% clobetasol cream in the treatment of chronic hand eczema: a protocol for systematic review and meta-analysis. Medicine 100:e24418
Promelle V, Goeb V, Gueudry J (2021) Rheumatoid arthritis Associated Episcleritis and Scleritis: an update on treatment perspectives, Journal of clinical medicine. 10
Verheijen EJA, Bonke CA, Amorij EMJ, Vleggeert-Lankamp CLA (2021) Epidural steroid compared to placebo injection in sciatica: a systematic review and meta-analysis, european spine journal: official publication of the european spine Society, the european spinal deformity Society, and the european section of the cervical spine Research Society
Cushing K, Higgins PDR (2021) Management of Crohn Disease: a review. Jama 325:69–80
Ruiz-Irastorza G, Bertsias G (2020) Treating systemic lupus erythematosus in the 21st century: new drugs and new perspectives on old drugs. Rheumatol (Oxford Engl 59:v69–v81
Deeb O, Nabulsi M (2020) Exploring multiple sclerosis (MS) and amyotrophic lateral scler osis (ALS) as neurodegenerative Diseases and their treatments: a review study. Curr Top Med Chem 20:2391–2403
Sood S, Bhatia GK, Seth P, Kumar P, Kaur J, Gupta V, Punia S, Tuli HS (2021) Efficacy and safety of New and emerging drugs for COVID-19: Favipiravir and Dexamethasone, Current pharmacology reports, 1–6
Mauvais-Jarvis F, Klein SL, Levin ER (2020) Estradiol, progesterone, Immunomodulation, and COVID-19 outcomes, Endocrinology. 161
Noreen S, Maqbool I, Madni A (2021) Dexamethasone: therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol 894:173854
Ahmed MH, Hassan A (2020) Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review, SN comprehensive clinical medicine, 1–10
Chen WJ, Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH (2021) Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/IGF1R-mediated EMT program. J food drug Anal 29:98–112
Fremont-Smith M (1946) The carcinogenic and Carcinostatic Effects of Estrogens on breast tissue. Trans Am Clin Climatological Association 58:61–70
Silberberg M, Silberberg R (1948) Age factor in estrogen-induced breast cancers of mice, Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 69, 438 – 41
Jordan VC, Koerner S (1975) Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J cancer 11:205–206
Fromson JM, Pearson S, Bramah S (1973) The metabolism of tamoxifen (I.C.I. 46,474). I. In laboratory animals. Xenobiotica; the fate of foreign compounds in biological systems 3:693–709
Jordan VC (2021) 50th anniversary of the first clinical trial with ICI 46,474 (tamoxifen): then what happened? Endocrine-related cancer 28:R11–r30
Wang L, Sharma A (2020) The Quest for Orally Available Selective Estrogen Receptor Degraders (SERDs), ChemMedChem 15, 2072–2097
Rochefort H, Bardon S, Chalbos D, Vignon F (1984) Steroidal and nonsteroidal antiestrogens in breast cancer cells in culture. J steroid Biochem 20:105–110
Scherbakov AM, Krasil’nikov MA, Kushlinskii NE (2013) Molecular mechanisms of hormone resistance of breast cancer. Bull experimental biology Med 155:384–395
Osborne CK, Boldt DH, Clark GM, Trent JM (1983) Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase. Cancer Res 43:3583–3585
Mukherjee AG, Wanjari UR, Nagarajan D, Vibhaa KK, Anagha V, Joshua Paul P, Tharani Priya T, Chakraborty R, Renu K, Dey A, Vellingiri B, Gopalakrishnan AV (2022) Letrozole: Pharmacology, toxicity and potential therapeutic effects. Life Sci 310:121074
Brodie AM, Coombes RC, Dowsett M (1987) Aromatase inhibitors: basic and clinical studies. J steroid Biochem 27:899–903
Brandt ME, Puett D, Garola R, Fendl K, Covey DF, Zimniski SJ (1988) Aromatase and aromatase inhibitors: from enzymology to selective chemotherapy. Progress in clinical and biological research 262:65–84
Cocconi G (1994) First generation aromatase inhibitors–aminoglutethimide and testololactone. Breast cancer research and treatment 30:57–80
Sciarra F (1988) Anti-estrogens and aromatase inhibitors: tamoxifen and testolactone. J endocrinological Invest 11:755–762
Ratre P, Mishra K, Dubey A, Vyas A, Jain A, Thareja S (2020) Aromatase inhibitors for the treatment of breast Cancer: a journey from the scratch. Anti-cancer agents in medicinal chemistry 20:1994–2004
DeLeve LD (2013) Chap. 30 - Cancer chemotherapy. In: Kaplowitz N, DeLeve LD (eds) Drug-Induced Liver Disease (Third Edition). Academic Press, Boston, pp 541–567
Wein AJ, Murphy JJ (1973) Experience in the treatment of prostatic carcinoma with cyproterone acetate. J Urol 109:68–70
Jabeen M, Choudhry MI, Miana GA, Rahman KM, Rashid U, Khan HU, Arshia, Sadiq A (2018) Synthesis, pharmacological evaluation and docking studies of progesterone and testosterone derivatives as anticancer agents. Steroids 136:22–31
Cabeza M, Bratoeff E, Heuze I, Rojas A, Terán N, Ochoa M, Ramírez-Apan T, Ramírez E, Pérez V, Gracia I (2006) New progesterone derivatives as inhibitors of 5alpha-reductase enzyme and prostate cancer cell growth. J enzyme Inhib Med Chem 21:371–378
Jonat W, Bachelot T, Ruhstaller T, Kuss I, Reimann U, Robertson JFR (2013) Randomized phase II study of lonaprisan as second-line therapy for progesterone receptor-positive breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology 24:2543–2548
Cabeza M, Heuze Y, Sánchez A, Garrido M, Bratoeff E (2015) Recent advances in structure of progestins and their binding to progesterone receptors. J enzyme Inhib Med Chem 30:152–159
Robertson JF, Willsher PC, Winterbottom L, Blamey RW, Thorpe S (1999) Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur J cancer 35:214–218
Scherbakov AM, Zavarzin IV, Vorontsova SK, Hajra A, Andreeva OE, Yadykov AV, Levina IS, Volkova YA, Shirinian VZ (2018) Synthesis and evaluation of the antiproliferative activity of benzylidenes of 16-dehydroprogesterone series. Steroids 138:91–101
Vorontsova SK, Yadykov AV, Scherbakov AM, Minyaev ME, Zavarzin IV, Mikhaevich EI, Volkova YA, Shirinian VZ (2020) Novel d-Annulated Pentacyclic Steroids: Regioselective Synthesis and Biological Evaluation in Breast Cancer Cells, Molecules (Basel, Switzerland) 25
Vorontsova SK, Zavarzin IV, Shirinian VZ, Bozhenko EI, Andreeva OE, Sorokin DV, Scherbakov AM, Minyaev ME (2022) Synthesis and crystal structures of D-annulated pentacyclic steroids: looking within and beyond AR signalling in prostate cancer. CrystEngComm 24:2089–2099
Iselt M, Holtei W, Hilgard P (1989) The tetrazolium dye assay for rapid in vitro assessment of cytotoxicity. Arzneimittelforschung 39:747–749
Volkova YA, Antonov YS, Komkov AV, Scherbakov AM, Shashkov AS, Menchikov LG, Chernoburova EI, Zavarzin IV (2016) Access to steroidal pyridazines via modified thiohydrazides. RSC Adv 6:42863–42868
Shchegolev Y, Sorokin D, Scherbakov A, Shunaev A, Andreeva O, Mikhaevich E, Gudkova M, Bure I, Berstein L, Nemtsova M, Krasil’nikov M (2020) Upregulation of Akt/Raptor signaling is associated with rapamycin resistance of breast cancer cells. Chemico-biological Interact 330:109243
Scherbakov AM, Stasevich OV, Salnikova DI, Andreeva OE, Mikhaevich EI (2020) Antiestrogenic and antiproliferative potency of secoisolariciresinol diglucoside derivatives on MCF-7 breast cancer cells, Natural product research, 1–7
Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F (2003) Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol cell 11:695–707
Scherbakov AM, Lobanova YS, Shatskaya VA, Onopchenko OV, Gershtein ES, Krasil’nikov MA (2006) Activation of mitogenic pathways and sensitization to estrogen-induced apoptosis: two independent characteristics of tamoxifen-resistant breast cancer cells? Breast cancer research and treatment 100:1–11
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Mruk DD, Cheng CY (2011) Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis 1:121–122
Piven YA, Yastrebova MA, Khamidullina AI, Scherbakov AM, Tatarskiy VV, Rusanova JA, Baranovsky AV, Zinovich VG, Khlebnicova TS, Lakhvich FA (2022) Novel O-acylated (E)-3-aryl-6,7-dihydrobenzisoxazol-4(5H)-one oximes targeting HSP90-HER2 axis in breast cancer cells. Bioorg Med Chem 53:116521
Kapara A, Findlay Paterson KA, Brunton VG, Graham D, Zagnoni M, Faulds K (2021) Detection of estrogen receptor alpha and Assessment of Fulvestrant activity in MCF-7 Tumor Spheroids using Microfluidics and SERS. Anal Chem 93:5862–5871
Wakeling AE, Bowler J (1992) ICI 182,780, a new antioestrogen with clinical potential. J steroid Biochem Mol biology 43:173–177
Brooks SC, Locke ER, Soule HD (1973) Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem 248:6251–6253
Gallo MA, Kaufman D (1997) Antagonistic and agonistic effects of tamoxifen: significance in human cancer. Seminars in oncology 24:S1–71
Fan P, Wang J, Santen RJ, Yue W (2007) Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67:1352–1360
Viedma-Rodríguez R, Baiza-Gutman L, Salamanca-Gómez F, Diaz-Zaragoza M, Martínez-Hernández G, Esparza-Garrido R, Velázquez-Flores R, M. A., Arenas-Aranda D (2014) Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review). Oncol Rep 32:3–15
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Li Z, Huang Y, Dong F, Li W, Ding L, Yu G, Xu D, Yang Y, Xu X, Tong D (2012) Swainsonine promotes apoptosis in human oesophageal squamous cell carcinoma cells in vitro and in vivo through activation of mitochondrial pathway. J Biosci 37:1005–1016
Kumari R, Ray AG, Mukherjee D, Chander V, Kar D, Kumar US, Bharadwaj PVPD, Banerjee SK, Konar A, Bandyopadhyay A (2022) Downregulation of PTEN promotes Autophagy via Concurrent reduction in apoptosis in Cardiac Hypertrophy in PPAR α(-/-) mice. Front Cardiovasc Med 9:798639
Cheng TC, Sayseng JO, Tu SH, Juan TC, Fang CL, Liao YC, Chu CY, Chang HW, Yen Y, Chen LC, Ho YS (2021) Curcumin-induced antitumor effects on triple-negative breast cancer patient-derived xenograft tumor mice through inhibiting salt-induced kinase-3 protein. J food drug Anal 29:622–637
Sabbah M, Courilleau D, Mester J, Redeuilh G (1999) Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci U S A 96:11217–11222
Ormerod MG, Collins MK, Rodriguez-Tarduchy G, Robertson D (1992) Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J immunological methods 153:57–65
Pellicciari C, Manfredi AA, Bottone MG, Schaack V, Barni S (1993) A single-step staining procedure for the detection and sorting of unfixed apoptotic thymocytes. Eur J histochemistry: EJH 37:381–390
Ling YH, el-Naggar AK, Priebe W, Perez-Soler R (1996) Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol Pharmacol 49:832–841
Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P (2007) Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating akt and p38 MAPK. Cancer biology & therapy 6:178–184
Iyengar M, O’Hayer P, Cole A, Sebastian T, Yang K, Coffman L, Buckanovich RJ (2018) CDK4/6 inhibition as maintenance and combination therapy for high grade serous ovarian cancer. Oncotarget 9:15658–15672
Pan Z, Zhang X, Yu P, Chen X, Lu P, Li M, Liu X, Li Z, Wei F, Wang K, Zheng Q, Li D (2019) Cinobufagin induces cell cycle arrest at the G2/M phase and promotes apoptosis in malignant melanoma cells. Front Oncol 9:853
Zhang M, Qu J, Gao Z, Qi Q, Yin H, Zhu L, Wu Y, Liu W, Yang J, Huang X (2020) Timosaponin AIII induces G2/M arrest and apoptosis in breast Cancer by activating the ATM/Chk2 and p38 MAPK signaling pathways. Front Pharmacol 11:601468
Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF (2000) Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog 28:102–110
Acknowledgements
Graphical abstract was created using Servier Medical Art templates. Original templates are licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/). The authors would like to thank Falcon Scientific Editing (https://falconediting.com) for proofreading the English language in this paper and the Center for Precision Genome Editing and Genetic Technologies for Biomedicine, IGB RAS, for providing The Beckman Coulter CytoFLEX flow cytometer. The study was supported by the Russian Science Foundation (project 19-15-00245, https://rscf.ru/project/22-15-35008/, experiments with resistant breast cancer cells). The authors thank M.A. Krasil’nikov for kind discussion and suggestions during correction of this manuscript.
Funding
The Russian Science Foundation (project 19-15-00245, https://rscf.ru/project/22-15-35008/, experiments with resistant breast cancer cells).
Author information
Authors and Affiliations
Author notes
Alexander M. Scherbakov and Svetlana K. Vorontsova contributed equally to this article.
- Alexander M. Scherbakov
Contributions
Conceptualization: A.M. Scherbakov and V.Z. Shirinian.
Contributed materials and critically revised the manuscript: A.M. Scherbakov, V. Jurisic and V.Z. Shirinian.
Performed the experiments, synthesized the compounds, analyzed all the data and amended the revised version: A.M. Scherbakov, S.K. Vorontsova, O.E. Andreeva, A.I. Khamidullina, J. Mrdjanovic, F.B. Bogdanov, D.I. Salnikova, V.Z. Shirinian.
Conceived and designed the experiments: A.M. Scherbakov, A.I. Khamidullina and V.Z. Shirinian.
Read, improved and approved the final manuscript: A.M. Scherbakov, S.K. Vorontsova, O.E. Andreeva, F.B. Bogdanov, D.I. Salnikova, A.I. Khamidullina, V. Jurisic, J. Mrdjanovic, I.V. Zavarzin, V.Z. Shirinian.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scherbakov, A.M., Vorontsova, S.K., Khamidullina, A.I. et al. Novel pentacyclic derivatives and benzylidenes of the progesterone series cause anti-estrogenic and antiproliferative effects and induce apoptosis in breast cancer cells. Invest New Drugs 41, 142–152 (2023). https://doi.org/10.1007/s10637-023-01332-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01332-z