Abstract
Purpose
To compare functional abnormalities of enhanced S-cone syndrome (ESCS), as examined using standard and extended electroretinography (ERG), with structural findings and retinal architecture obtained by spectral domain optical coherence tomography (SD-OCT).
Methods
Four patients with ESCS underwent standard full-field and multifocal ERGs, with extended S-cone and ON/OFF ERG protocols also performed. SD-OCT was also carried out, and longitudinal reflectivity profiles (LRPs) were calculated for the perifoveolar retina.
Results
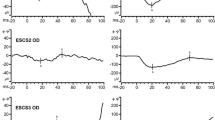
All four patients exhibited pathognomonic full-field ERG findings for ESCS, with delayed responses of similar waveforms to the same intensity flash under both scotopic and photopic conditions. The amplitudes of the full-field ERGs showed considerable variation between patients, which were not related to the extent of the visual field defects. Multifocal ERGs reflected preserved central function in eyes with good visual acuity (Snellen visual acuity >0.7). The ERGs to S-cone-specific stimulation confirmed the expected predominant activity of the S-cone system in all four patients. The ON/OFF ERG recordings revealed abnormal presence of both ON-response and OFF-response activities in three patients; the remaining patient showed only OFF-response activity. SD-OCT showed a significantly thickened outer nuclear layer in all four patients, as obtained by LRP analysis. Furthermore, in the patient with selective preservation of the OFF-response activity, LRP showed reduced numbers of hyper-reflectivity sub-peaks in the inner plexiform layer.
Conclusion
Patients with ESCS show characteristic full-field ERG waveform abnormality, predominance of S-cone ERG activity, and thickening of the outer nuclear layer on SD-OCT. Moreover, they can also show abnormal post-photoreceptor connectivity through S-cone-related OFF-bipolar cell activity.





Similar content being viewed by others
References
Jacobson SG, Marmor MF, Kemp CM, Knighton RW (1990) SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31:827–838
Marmor MF, Jacobson SG, Foerster MH et al (1990) Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol 110:124–134
Kobayashi M, Takezawa S, Hara K et al (1999) Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci USA 96:4814–4819
Haider NB, Jacobson SG, Cideciyan AV et al (2000) Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 24:127–131
Schorderet DF, Escher P (2009) NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum Mutat 30:1475–1485
Milam AH, Rose L, Cideciyan AV et al (2002) The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci USA 99:473–478
Sharon D, Sandberg MA, Caruso RC et al (2003) Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol 121:1316–1323
Audo I, Michaelides M, Robson AG et al (2008) Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 49:2082–2093
Marmor MF (1989) Large rod-like photopic signals in a possible new form of congenital night blindness. Doc Ophthalmol 71:265–269
Udar N, Small K, Chalukya M et al (2011) Developmental or degenerative–NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol Vis 17:519–525
Lam BL, Goldberg JL, Hartley KL et al (2007) Atypical mild enhanced S-cone syndrome with novel compound heterozygosity of the NR2E3 gene. Am J Ophthalmol 144:157–159
Kinori M, Pras E, Kolker A et al (2011) Enhanced S-cone function with preserved rod function: a new clinical phenotype. Mol Vis 17:2241–2247
Marmor MF, Tan F, Sutter EE, Bearse MA (1999) Topography of cone electrophysiology in the enhanced S cone syndrome. Invest Ophthalmol Vis Sci 40:1866–1873
Román AJ, Jacobson SG (1991) S cone-driven but not S cone-type electroretinograms in the enhanced S cone syndrome. Exp Eye Res 53:685–690
Miyake Y (ed) (2006) Enhanced S-cone syndrome. In: Electrodiagnosis of retinal diseases. Springer, Tokyo, pp 68–71
Gouras P (2003) The role of S-cones in human vision. Doc Ophthalmol 106:5–11
Jacobson SG, Sumaroka A, Aleman TS et al (2004) Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual degeneration. Hum Mol Genet 13:1893–1902
Hayashi T, Kitahara K (2005) Optical coherence tomography in enhanced S-cone syndrome: large macular retinoschisis with disorganized retinal lamination. Eur J Ophthalmol 15:643–646
Park SP, Hong IH, Tsang SH et al (2013) Disruption of the human cone photoreceptor mosaic from a defect in NR2E3 transcription factor function in young adults. Graefes Arch Clin Exp Ophthalmol 251:2299–2309
Cima I, Brecelj J, Sustar M et al (2012) Enhanced S-cone syndrome with preserved macular structure and severely depressed retinal function. Doc Ophthalmol 125:161–168
Sustar M, Hawlina M, Brecelj J (2011) Electroretinographic evaluation of the retinal S-cone system. Doc Ophthalmol 123:199–210
Beharic A, Stirn-Kranjc B, Brecelj J (2012) Electrophysiological assessment of the retina in children with congenital nystagmus. Zdr Vest 81:73–82
Sustar M, Stirn-Kranjc B, Brecelj J (2012) Children with complete or incomplete congenital stationary night blindness: ophthalmological findings, standard ERGs and ON-OFF ERGs for differentiation between the types. Zdr Vest 81:16–28
Hawlina M, Konec B (1992) New noncorneal HK-loop electrode for clinical electroretinography. Doc Ophthalmol 81:253–259
Hood DC, Bach M, Brigell M et al (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124:1–13
Marmor MF, Fulton AB, Holder GE et al (2009) ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118:69–77
Barthelmes D, Gillies MC, Sutter FKP (2008) Quantitative OCT analysis of idiopathic perifoveal telangiectasia. Invest Ophthalmol Vis Sci 49:2156–2162
Barthelmes D, Sutter FK, Kurz-Levin MM et al (2006) Quantitative analysis of OCT characteristics in patients with achromatopsia and blue-cone monochromatism. Invest Ophthalmol Vis Sci 47:1161–1166
Hood DC, Cideciyan AV, Roman AJ, Jacobson SG (1995) Enhanced S cone syndrome: evidence for an abnormally large number of S cones. Vision Res 35:1473–1481
Greenstein VC, Zaidi Q, Hood DC et al (1996) The enhanced S cone syndrome: an analysis of receptoral and post-receptoral changes. Vis Res 36:3711–3722
Bandah D, Merin S, Ashhab M et al (2009) The spectrum of retinal diseases caused by NR2E3 mutations in Israeli and Palestinian patients. Arch Ophthalmol 127:297–302
Iannaccone A, Fung KH, Eyestone ME, Stone EM (2009) Treatment of adult-onset acute macular retinoschisis in enhanced S-cone syndrome with oral acetazolamide. Am J Ophthalmol 147:307–312
Pachydaki SI, Klaver CC, Barbazetto IA et al (2009) Phenotypic features of patients with NR2E3 mutations. Arch Ophthalmol 127:71–75
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Sieving PA (1993) Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91:701–773
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532
Ueno S, Kondo M, Ueno M et al (2006) Contribution of retinal neurons to d-wave of primate photopic electroretinograms. Vis Res 46:658–664
Evers HU, Gouras P (1986) Three cone mechanisms in the primate electroretinogram: two with, one without off-center bipolar responses. Vis Res 26:245–254
Tanna H, Dubis AM, Ayub N et al (2010) Retinal imaging using commercial broadband optical coherence tomography. Br J Ophthalmol 94:372–376
Famiglietti EV, Kolb H (1976) Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science 194:193–195
Nelson R, Famiglietti EV, Kolb H (1978) Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol 41:472–483
Acknowledgments
The authors are grateful to Mrs. Marija Jesenšek and Mrs. Ana Jeršin, who were involved in the clinical ERG recording. This study was supported by the Slovenian Research Agency, Grant No. P3-0333. All of the authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest, in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sustar, M., Perovšek, D., Cima, I. et al. Electroretinography and optical coherence tomography reveal abnormal post-photoreceptoral activity and altered retinal lamination in patients with enhanced S-cone syndrome. Doc Ophthalmol 130, 165–177 (2015). https://doi.org/10.1007/s10633-015-9487-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-015-9487-9