Abstract
Background and Aims
Acute pancreatitis (AP) is an acute inflammatory disease that can lead to death. Mir-325-3p is strongly and abnormally expressed in many diseases, necessitating exploration of its function and mechanism in AP.
Methods
Blood samples from AP patients and mice were analyzed. The expression levels of miR-325-3p in AP patients and mouse were detected. Whether miR-325-3p targets RIPK3 gene was predicted by TargetScan online database and dual luciferase reporter assay. In vitro experiments verified the effect of miR-325-3p overexpression on caerulein-induced MPC83 pancreatic acinar cancer cell line. In vivo experiments verified the effect of overexpression of miR-325-3p on the disease degree of pancreatic tissues in AP mice.
Results
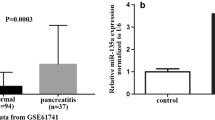
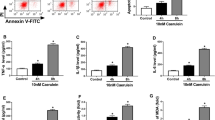
Analysis of blood samples from AP patients and experiments in mice demonstrated that expression of miR-325-3p was significantly reduced during the process of AP in humans and mice. Predicted using the TargetScan online database and through dual luciferase reporter assay detection, miR-325-3p directly targets the RIPK3 gene. In vitro experiments revealed that overexpression of miR-325-3p reversed caerulein-induced apoptosis and necroptosis in MPC83 pancreatic acinar cancer cell line. We used Z-VAD-FMK to assess necroptosis and demonstrated that miR-325-3p targets necroptosis to reduce cell damage. In subsequent experiments in mice, we verified that overexpression of miR-325-3p reduces inflammation, edema, hemorrhage, and necrosis in acute pancreatitis. Characteristic western blot, immunohistochemistry, and transmission electron microscopy results revealed that overexpression of miR-325-3p reduces the severity of acute pancreatitis by inhibiting pancreatic necroptosis in AP mice.
Conclusions
The current research results indicate that miR-325-3p directly targets RIPK3 and exerts a protective role in mouse AP. Necroptosis is still the primary mechanism of RIPK3 regulation. MiR-325-3p inhibits acute pancreatitis by targeting RIPK3-dependent necroptosis, which may represent a novel treatment method for acute pancreatitis.







Similar content being viewed by others
References
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019;16:175–184.
Leppäniemi A, Tolonen M, Tarasconi A et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg 2019;14:27.
Barreto SG, Habtezion A, Gukovskaya A et al. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut 2021;70:194–203.
Roberts SE, Morrison-Rees S, John A et al. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017;17:155–165.
Shabanzadeh DM, Novovic S. Alcohol, smoking and benign hepato-biliary disease. Best Pract Res Clin Gastroenterol 2017;31:519–527.
Boxhoorn L, Voermans RP, Bouwense SA et al. Acute pancreatitis. Lancet 2020;396:726–734.
Ohmuraya M, Yamamura K. Autophagy and acute pancreatitis: a novel autophagy theory for trypsinogen activation. Autophagy 2008;4:1060–1062.
Huang Z, Ma X, Jia X et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: a randomized controlled clinical trial. Am J Gastroenterol 2020;115:473–480.
Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2004;286:G189–G196.
Bai Y, Lam HC, Lei X. Dissecting programmed cell death with small molecules. Acc Chem Res 2020;53:1034–1045.
Kang R, Lotze MT, Zeh HJ et al. Cell death and DAMPs in acute pancreatitis. Mol Med 2014;20:466–477.
Galluzzi L, Kepp O, Chan FK et al. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol 2017;12:103–130.
Wang G, Qu FZ, Li L et al. Necroptosis: a potential, promising target and switch in acute pancreatitis. Apoptosis 2016;21:121–129.
Wu J, Huang Z, Ren J et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 2013;23:994–1006.
Newton K, Dugger DL, Maltzman A et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ 2016;23:1565–1576.
Hong YP, Yu J, Su YR et al. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxid Med Cell Longev 2020;2020:8172714.
Kearney CJ, Cullen SP, Tynan GA et al. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ 2015;22:1313–1327.
Boonchan M, Arimochi H, Otsuka K et al. Necroptosis protects against exacerbation of acute pancreatitis. Cell Death Dis 2021;12:601.
Louhimo J, Steer ML, Perides G. Necroptosis is an important severity determinant and potential therapeutic target in experimental severe pancreatitis. Cell Mol Gastroenterol Hepatol 2016;2:519–535.
Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11:597–610.
Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203–222.
Liu P, Xia L, Zhang WL et al. Identification of serum microRNAs as diagnostic and prognostic biomarkers for acute pancreatitis. Pancreatology 2014;14:159–166.
Bo L, Su-Ling D, Fang L et al. Autophagic program is regulated by miR-325. Cell Death Differ 2014;21:967–977.
Wang H, Hu X, Yang F, et al. miR-325-3p promotes the proliferation, invasion and EMT of breast cancer cells by directly targeting S100A2. Oncol Res. 2021.
Chen X, Gao J, Yu Y et al. LncRNA FOXD3-AS1 promotes proliferation, invasion and migration of cutaneous malignant melanoma via regulating miR-325/MAP3K2. Biomed Pharmacother 2019;120:109438.
Fu B, Xue W, Zhang H, et al. MicroRNA-325–3p facilitates immune escape of mycobacterium tuberculosis through targeting LNX1 via NEK6 accumulation to promote anti-apoptotic STAT3 signaling. mBio. 2020;11.
Gu E, Pan W, Chen K et al. LncRNA H19 regulates lipopolysaccharide (LPS)-induced apoptosis and inflammation of BV2 microglia cells through targeting miR-325-3p/NEUROD4 axis. J Mol Neurosci 2021;71:1256–1265.
Zhang L, Liu SY, Yang X et al. TMEM206 is a potential prognostic marker of hepatocellular carcinoma. Oncol Lett 2020;20:174.
Lin T, Zhou S, Gao H et al. MicroRNA-325 is a potential biomarker and tumor regulator in human bladder cancer. Technol Cancer Res Treat 2018;17:1533033818790536.
Wan J, Yang X, Ren Y et al. Inhibition of miR-155 reduces impaired autophagy and improves prognosis in an experimental pancreatitis mouse model. Cell Death Dis 2019;10:303.
Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 2013;144:1180–1193.
Cui L, Liu R, Li C et al. Angiotensin-(1–7) attenuates caerulein-induced pancreatic acinar cell apoptosis. Mol Med Rep 2017;16:3455–3460.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015;517:311–320.
Zhang DW, Shao J, Lin J et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009;325:332–336.
Mandal P, Berger SB, Pillay S et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 2014;56:481–495.
Feoktistova M, Geserick P, Kellert B et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011;43:449–463.
Lawlor KE, Khan N, Mildenhall A et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 2015;6:6282.
Cho YS, Challa S, Moquin D et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009;137:1112–1123.
Moriwaki K, Balaji S, McQuade T et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 2014;41:567–578.
Wang Q, Liu Z, Ren J et al. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res 2015;116:600–611.
Acknowledgments
We thank all members of the laboratory for useful discussion.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
A.J. and ZW.Y. were involved in the conceptualization; A.J. contributed to the methodology; A.J. and JM.L. contributed to the software; JY.S. and K.Z. were involved in the validation; JM.L. contributed to the investigation; JY.S. contributed to resources; A.J. was involved in the data curation; A.J. was involved in the writing—original draft preparation; ZW.Y. contributed to the writing—review and editing; A.J. was involved in the visualization; YF.C. was involved in the supervision; YF.C. was involved in the project administration; YF.C acquired the funding. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in this work.
Ethical statement
Institutional Review Board Statement: This study was approved by the Ethics Committee of Nankai Hospital and was performed in accordance with the Helsinki Declaration of Principles (ethics review item number: NKYY_YXKT_IRB_2020_059_01). All animal experiments were authorized by the Animal Ethics Committee of Tianjin Nankai Hospital. The ethical review item number is NKYY-DWLL-2020-115, and this study was performed in accordance with the National Laboratory Animal Care and Use Guide.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, A., Yang, ZW., Shi, JY. et al. MiR-325-3p Alleviates Acute Pancreatitis via Targeting RIPK3. Dig Dis Sci 67, 4471–4483 (2022). https://doi.org/10.1007/s10620-021-07322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07322-6